Literature Sharing: Multiplex Immunofluorescence Combined with In situ Hybridization for Analysis of Animal Tissue Immunopathology
I. Research Background
Tissue as a key network constituting organs, organ systems and coordinated biological functions of organisms, its in-situ state analysis is crucial for understanding and regulating biological outcomes affecting animal health. However, in the field of animal medicine, in-situ research has long been limited by the scarcity of species-compatible immunoreagents——the availability of immunoreagents targeting specific cells, functions or pathological markers is extremely low, and even when relevant reagents are found, they often can only achieve simultaneous detection of single or few markers, making it difficult to form a comprehensive biological interpretation of tissue status.
Based on this core bottleneck, the research "In situ staining with antibodies cross-reactive in pigs, cattle, and white-tailed deer facilitates understanding of biological tissue status and immunopathology" developed a multiplex chromogenic immunohistochemistry scheme for comparative study of immune cells in lymph nodes across species, and verified the ability of fluorescent multiplex staining to detect protein co-localization signals in porcine tissues. Given the scarcity and preparation difficulty of protein-reactive antibodies in animals, the research further proposed to design immunoreagents targeting in-situ targets through RNA level design and combine them with species antibody staining in multiplex combinations to break through the limitations of immunoreagents in in-situ detection. To confirm the role of combined RNA and protein detection in elucidating tissue status and immunopathology, the research achieved combined staining of cell-specific protein markers with Seneca virus A (SVA) RNA in porcine tonsil tissue.
II. Research Methods
Tissue samples were fixed with 10% neutral buffered formalin for approximately 24 hours, followed by routine dehydration, paraffin embedding and sectioning. Antibodies targeting 5 key markers were screened, including: macrophage/dendritic cell marker IBA-1, T cell marker CD3, B cell marker Pax5, proliferating cell marker Ki-67, epithelial cell marker cytokeratin. Then the logic of single staining immunohistochemistry and multiplex staining immunohistochemistry verification, multiplex immunofluorescence staining verification, chromogenic method RNA in situ hybridization, multiplex immunofluorescence and RNA in situ hybridization co-detection verification was followed for experiments. After sample processing, incubation and mounting, fluorescence staining results were observed using Nikon A1R confocal microscopy. Multiplex immunofluorescence and RNA in situ hybridization co-detection first used RNAscope technology for RNA in situ hybridization, using SVA capsid protein 1 gene sequence as target probe design; protein and RNA co-detection was based on improved RNAscope multiplex fluorescence detection kit integrated with immunofluorescence co-detection workflow, detecting RNA signals through Opal 570, detecting protein signals through TSA 650 combined with secondary antibody. Antibody compatibility with RNAscope technology was first verified, then using tonsil tissue from SVA-infected pigs as samples to achieve co-detection of SVA RNA and cell marker proteins.
III. Results Analysis
IHC Detection of Markers in Animal Tissues
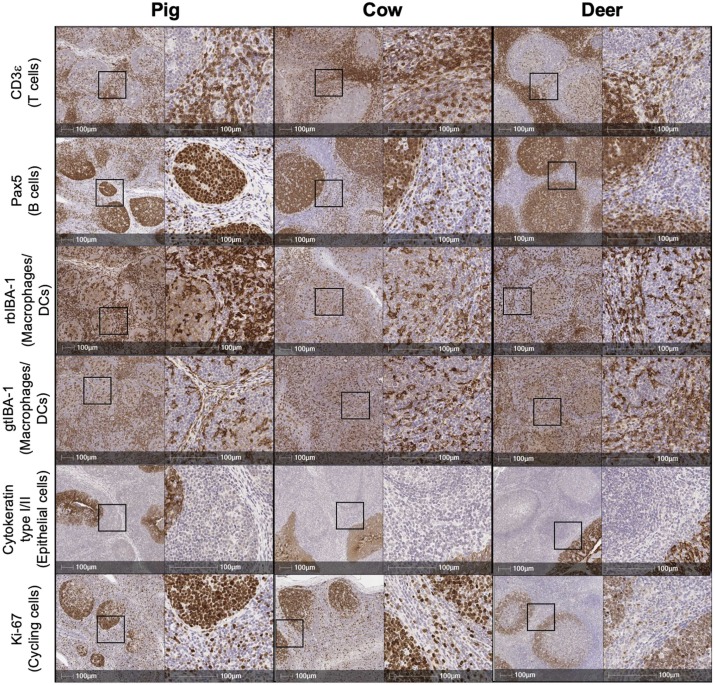
The core purpose of this figure is to verify the conserved reactivity of selected antibodies in FFPE tissues of three different veterinary-related species: pig, cattle, and deer. The experiment used palatine tonsil tissue as detection samples, showing positive staining signals of CD3, Pax5, IBA-1, gtIBA-1, cytokeratin, and Ki-67 in all three species through single IHC staining. All selected antibodies can specifically bind and develop color in target cells of all three species.
Multiplex IHC and Fluorescence Detection of Markers
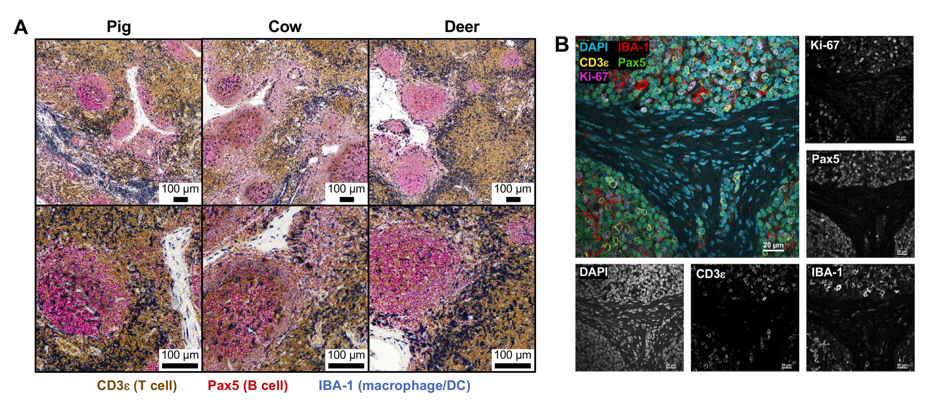
Figure A shows triple chromogenic IHC results, using tracheobronchial lymph node samples from pigs, cattle, and white-tailed deer to simultaneously detect CD3, Pax5, and IBA-1. Results showed that lymph node immune structures of all three species conform to expected histological features: paracortical areas dominated by CD3+ T cells, subcapsular, trabecular and medullary sinuses dominated by IBA-1+ macrophages/DC, and lymphoid follicles dominated by Pax5+ B cells. This result proves that triple chromogenic IHC can be effectively used for comparative analysis of lymph node immune structures across species.
Figure B shows quadruple immunofluorescence staining results, using porcine mesenteric lymph node samples to simultaneously detect IBA-1, CD3, Pax5, and Ki-67, and showing single-channel grayscale images of each marker. Results not only verified immune cell distribution patterns consistent with Figure A, but also achieved clear detection of co-localization signals, directly identifying IBA-1+ macrophages/DC, Pax5+ B cells and CD3+ T cells simultaneously expressing Ki-67, indicating the presence of proliferating immune cells. Moreover, most Ki-67+ cells co-localized with Pax5+ cells and were concentrated within lymphoid follicles, proving the existence of active germinal centers containing proliferating B cells.
Compatibility Verification of Antibody Staining with RNA In situ Hybridization
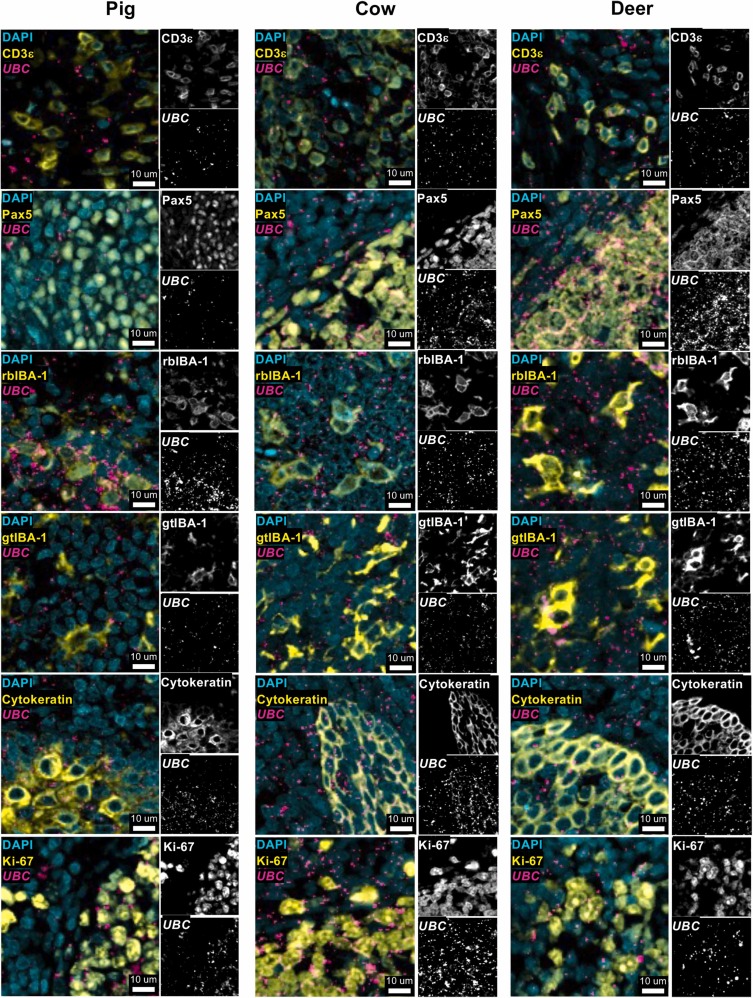
Using tonsil tissue samples from pigs, cattle, and white-tailed deer, the compatibility of selected antibodies with RNAscope RNA in situ hybridization technology was verified through fluorescence staining. The experiment simultaneously detected each protein marker with housekeeping gene UBC's RNA, and showed single-channel grayscale images of each marker. In all three species, protein staining signals of all tested antibodies could clearly co-localize with UBC RNA signals without obvious interference. This proves that selected antibodies are compatible with RNA in situ hybridization technology, providing a key basis for subsequent development of multiplex in-situ schemes for "protein-RNA co-detection".
Application of Protein-RNA Co-detection in Immunopathology Research
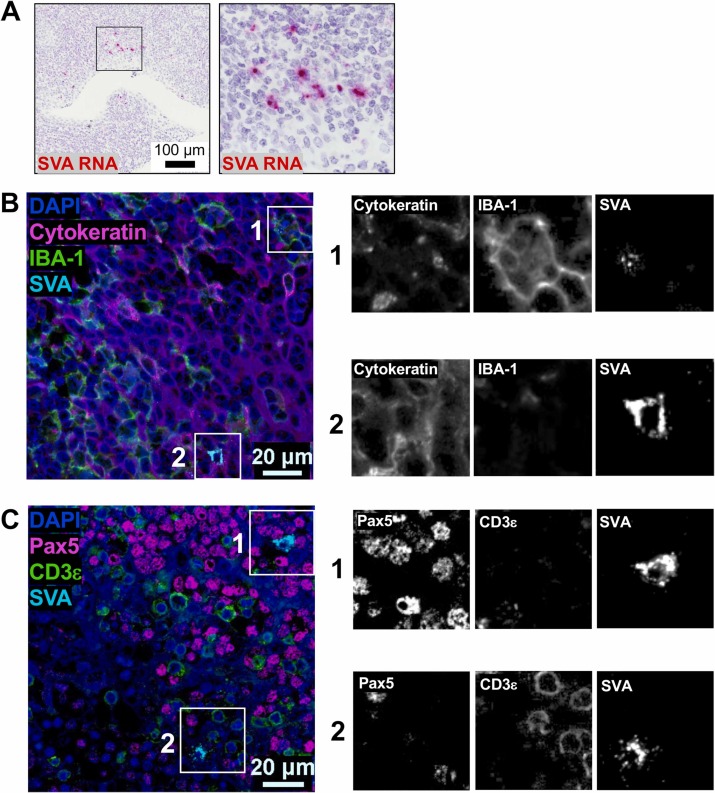
Using tonsil tissue from SVA-infected pigs as samples, the distribution of SVA-specific RNA in the tissue was first demonstrated through chromogenic display, showing that viral RNA was mainly concentrated in the subepithelial follicular interstitial parenchyma and surrounding areas, clarifying the infection localization area of the virus in the tissue. Then multiplex fluorescence staining simultaneously detected protein markers with SVA RNA, and showed single-channel grayscale images of key areas.Experimental results found that Seneca virus A 's RNA signals and target protein signals could precisely localize to the same cells. This not only proves that the combined detection method of "antibody detection + RNA in situ hybridization" is reliable and effective, but also clearly shows that this virus tends to infect which type of cells. This method provides a completely new technical approach for studying the immunopathological mechanisms of infectious diseases in the veterinary field.
IV. Summary
This research addresses the core pain points of scarce species-compatible immunoreagents and difficulty in multiplex marker detection in veterinary in-situ research. Through systematic screening and verification, we successfully established a set of multiplex in-situ staining techniques that can be used in multiple veterinary species including pigs, cattle, and deer, including brightfield multiplex IHC, fluorescence multiplex IHC, and protein-RNA integrated in-situ detection schemes. Moreover, selected antibodies have cross-species conserved reactivity, can accurately recognize target cell markers, multiplex staining methods can clearly tissue immune landscapes and cellular functional states, and protein and RNA co-detection can effectively identify pathogen infection target cell types. These research achievements not only solve technical difficulties in traditional veterinary in-situ research, but also provide standardized methodological tools for comparative studies of tissue characteristics and immunopathology between different animal species. At the same time, they also lay an important technical foundation for subsequent research on the causes of veterinary infectious diseases and screening of disease diagnostic markers.
References
Wiarda JE, Zanella EL, Shircliff AL, Cassmann ED, Loving CL, Buckley AC, Palmer MV. In situ staining with antibodies cross-reactive in pigs, cattle, and white-tailed deer facilitates understanding of biological tissue status and immunopathology. Vet Immunol Immunopathol. 2025 Jan;279:110865. doi: 10.1016/j.vetimm.2024.110865. Epub 2024 Dec 12. PMID: 39719720.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
