Literature Sharing: Multiplex Immunofluorescence for Early Breast Cancer ICI Therapeutic Efficacy Prediction
Background
In recent years, immune checkpoint inhibitors (ICIs) have shown potential value in breast cancer treatment, but treatment responses vary significantly among different patients. The characteristics of the tumor immune microenvironment (TIME) are key factors affecting the efficacy of ICIs. Currently, there is still a lack of systematic and precise definition of predictive biomarkers for PD-L1 inhibitor treatment response in breast cancer patients, especially the differences in treatment response mechanisms among different receptor subtypes of breast cancer have not been fully elucidated. New detection technologies developed in recent years can simultaneously evaluate multiple markers while completely preserving the spatial relationships of cells in situ, and are being widely applied to tumor immune microenvironment characterization and mining of immune-related biomarkers. Among these, multiplex immunofluorescence (mIF) technology has demonstrated application value in predicting ICI efficacy in various tumors, significantly outperforming gene expression signatures, tumor mutational burden, and standardized PD-L1 immunohistochemistry detection. Based on this background, the study "Multi-platform biomarkers of response to an immune checkpoint inhibitor in the neoadjuvant I-SPY 2 trial for early-stage breast cancer" analyzed the density and spatial distribution characteristics of immune cells in the tumor microenvironment of patients treated with neoadjuvant chemotherapy ± pembrolizumab through multiplex immunofluorescence detection. Combined with gene expression profiling and reverse protein array technology, it explored immune signaling pathways and biomarkers and pathway characteristics related to DNA repair defects, ultimately providing important evidence for clinical precision screening of ICI treatment-benefiting populations and optimization of early breast cancer immunotherapy strategies.
Research Methods
The Opal 7-color fluorescence immunohistochemistry kit was used to perform multiplex immunofluorescence staining on 4μm sections of pre-treatment formalin-fixed paraffin-embedded tumor tissue. The process involved heat-induced epitope retrieval (HIER), primary antibody incubation, HRP-labeled secondary antibody incubation, and tyramide signal amplification with Opal fluorophore development. Subsequent repeated staining workflows after HIER removal of bound antibodies were performed, followed by DAPI staining and mounting. Antibody labeling sequences were determined based on antigen stability to repeated HIER, and fluorescent dye matching balanced staining intensity with cellular localization to reduce signal crosstalk. Sections were imaged using a Vectra 3.0 scanner, with 15-20 multispectral images at 20x magnification collected per tumor biopsy sample. Spectral unmixing and cell segmentation were completed using inForm quantitative pathology software.To analyze spatial relationships between specific cell phenotypes, this study employed spatial point pattern analysis using R packages such as phenoptr, spatstat, and divo. Each multiplex immunofluorescence image was virtually divided into non-overlapping 200μm×200μm squares, and the numbers of cancer cells and immune cells within each square were counted. The Morisita-Horn (MH) similarity index from the divo R package was then used to calculate the degree of colocalization among 21 groups of cell types. The MH index ranges from 0 to 1, where 0 represents high separation between cell types A and B.
mIF Identification of Immune Cell Infiltration in Breast Cancer Tissue
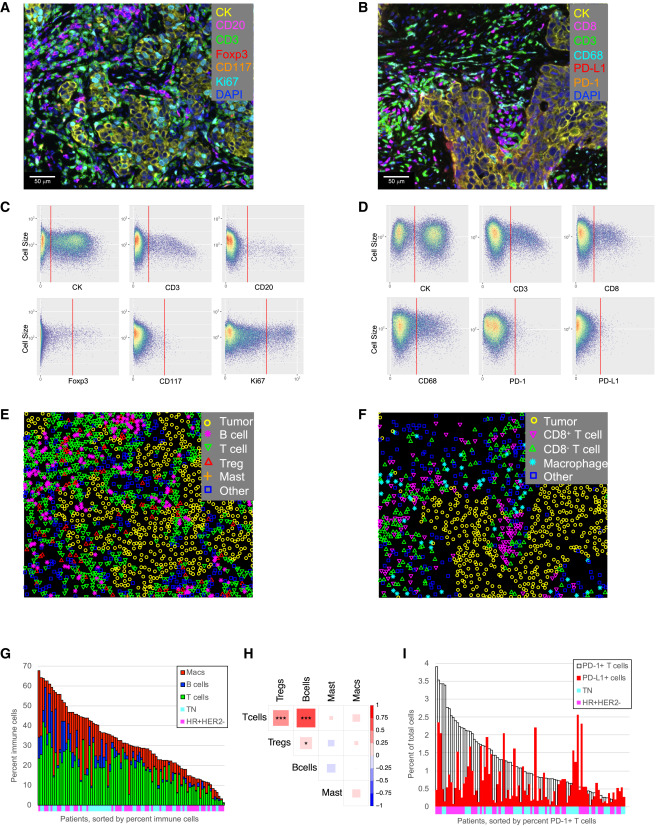
The study developed two multiplex immunofluorescence detection panels for analyzing immune cell infiltration in pre-treatment biopsy samples. Automated gating algorithms completed cell phenotype identification and generated phenotype maps for subsequent spatial analysis. Results showed significant differences in immune cell density among different tumors, with immune cells accounting for 1%-68% of total cells. Among these, T cells, B cells, and macrophages accounted for < 1%-42%, <1%-22%, and <1%-43% respectively. Significant correlations were found between T cells, B cells and Treg density, while macrophage and mast cell density showed no association with these lymphoid cell populations. Additionally, PD-1/PD-L1 staining levels also exhibited obvious heterogeneity, with PD-1+ T cells accounting for < 0.04%-3.9% of total cells, and PD-L1+ cells accounting for < 0.06%-2.6%.
Association of Immune Cell-Related Biomarkers with Treatment Response
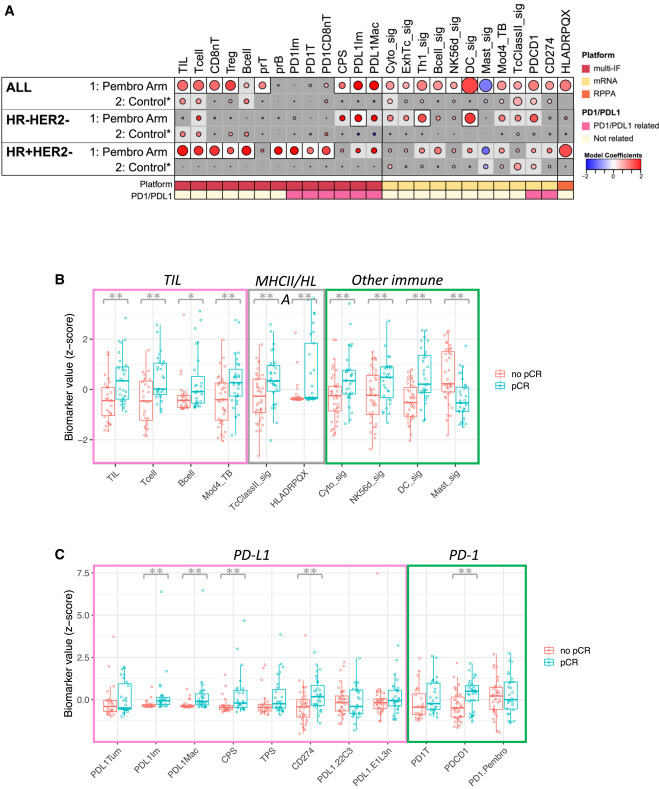
This study analyzed the predictive role of immune cell-related indicators on post-treatment pathological complete response (pCR) in different types of breast cancer patients in the pembrolizumab treatment group and conventional chemotherapy control group. Results showed that indicators predicting therapeutic efficacy in the pembrolizumab group were far more numerous than in the control group, and effective predictive indicators differed among different types of breast cancer. Specifically, among 20 immune cell-related indicators detected by multiplex immunofluorescence technology, 8 showed significant positive correlation with pembrolizumab treatment efficacy, including tumor-infiltrating lymphocytes (TILs), various T cells, two types of PD-L1-expressing cells, and PD-L1 CPS score. Higher values of these indicators were associated with greater probability of complete tumor disappearance, while in the control group, only TILs, T cells, and B cells showed weak associations with efficacy. Among the 18 gene-related indicators analyzed, 11 could only predict pembrolizumab treatment efficacy, covering gene signatures corresponding to various immune cells and encoding genes for PD-1 and PD-L1. Among 11 immune-related proteins detected by protein detection technology, only HLADRPQX protein was associated with pembrolizumab treatment efficacy. Among the overall 49 immune-related indicators, 20 could clearly predict immunotherapy efficacy, while the control group showed only 6 indicators with unclear associations with efficacy. Among these, dendritic cell-related gene signatures showed the best predictive efficacy, while mast cell-related gene signatures showed negative correlation with efficacy. In triple-negative breast cancer, 3 indicators could clearly predict immunotherapy efficacy and 8 indicators showed certain associations, mainly involving PD-L1-expressing immune cells and two gene signatures. In HR+HER2- breast cancer, 10 indicators could clearly predict efficacy and 9 indicators showed certain associations, including HLADRPQX protein and various immune cells. Importantly, these indicators predicting immunotherapy efficacy showed no association with treatment efficacy in their respective control groups.
Correlation of Immune Cell and Tumor Cell Colocalization with Pembrolizumab Response
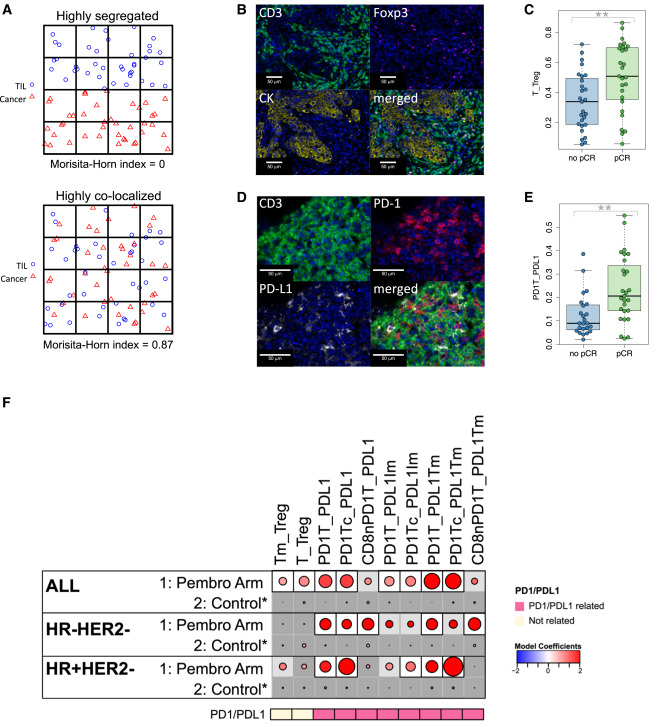
The study explored spatial proximity differences between responders and non-responders among various immune cell populations and tumor cells or other immune cells, using MH index and nearest neighbor distribution function for spatial proximity analysis. Results showed that MH index-related indicators were significantly associated with pembrolizumab treatment response and were concentrated in PD-1/PD-L1-related cell colocalization, showing subtype specificity.
Specifically, the MH index measures the degree of colocalization between two cell types, ranging from 0 to 1. When two cell types are highly colocalized, the index approaches 1, and when highly separated, it approaches 0. The study calculated MH indices for 21 groups of cell types. High MH indices between T cells and regulatory T cells (Tregs) were significantly associated with pembrolizumab treatment response in the overall cohort. High MH indices between PD-1+ T cells and PD-L1+ cells were also associated with pCR. After multiple testing correction, 8 of 21 MH indices were associated with pembrolizumab treatment response in the overall cohort but not with the control group. Among these, 6 involved PD-1/PD-L1-related cell colocalization, and 3 showed significant interactions with treatment. When analyzed by receptor subtype, 7 MH indices were positively correlated with pembrolizumab group pCR and unrelated to the control group. These indices were all related to PD-1/PD-L1-related cell colocalization and were highly correlated with each other. Among these, colocalization of PD-1+ T cells with PD-L1+ cells or PD-L1+ tumor cells, and colocalization of PD-1+ CD8+ cytotoxic T cells with PD-L1+ cells were associated with pCR in both triple-negative and HR+HER2- tumors.
Correlation of Spatial Proximity between Immune Cells and Tumor Cells with Pembrolizumab Response
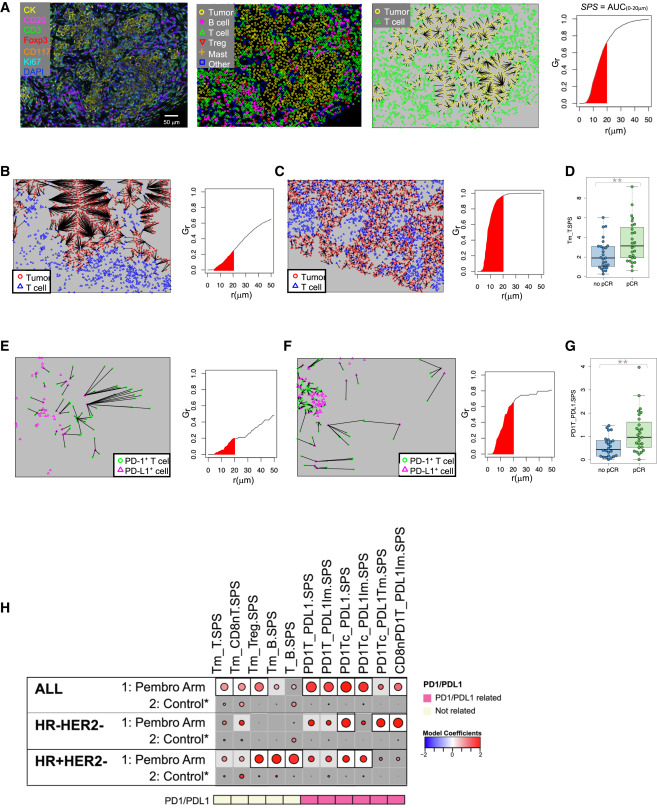
The study further investigated spatial proximity between immune cells and tumor cells using spatial point pattern analysis and nearest neighbor distribution functions. Results indicated that in pembrolizumab-treated patients, closer spatial proximity between certain immune cell populations and tumor cells was associated with improved treatment response. Specifically, spatial proximity between PD-1+ T cells and PD-L1+ tumor cells showed the strongest correlation with pCR, particularly in triple-negative breast cancer. Additionally, the study found that spatial proximity between dendritic cells and tumor cells was also associated with pembrolizumab treatment response, suggesting a potential role for antigen presentation in ICI efficacy. These findings highlight the importance of spatial relationships within the tumor immune microenvironment in determining treatment response to immune checkpoint inhibitors.
References
Campbell MJ, Wolf DM, Yau C, Brown-Swigart L, Wulfkuhle J, Gallagher IR, Zhu Z, Bolen J, Vandenberg S, Hoyt C, Mori H, Borowsky A, Sit L, Perlmutter J, Asare SM; I-SPY2 Investigators; Nanda R, Liu MC, Yee D, DeMichele AM, Hylton NM, Pusztai L, Berry DA, Hirst GL, Petricoin EF, Veer LV, Esserman L. Multi-platform biomarkers of response to an immune checkpoint inhibitor in the neoadjuvant I-SPY 2 trial for early-stage breast cancer. Cell Rep Med. 2024 Nov 19;5(11):101799. doi: 10.1016/j.xcrm.2024.101799. Epub 2024 Nov 6. PMID: 39510069; PMCID: PMC11604542.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
