Literature Share: Multiplexed Fluorescent Staining of Pulmonary Tuberculosis Lesions Based on TSA
Background
Mycobacterium tuberculosis (Mtb) is an obligate human pathogen that enters alveoli through aerosol transmission and induces the formation of primary lesions in resident alveolar macrophages and recruited myeloid cells, which then develop into granulomas with central necrosis characteristics — this is the typical pathological hallmark of tuberculosis. Mtb can evade host immune surveillance within granulomas and survive under stress environments such as hypoxia, low pH, and nutrient deprivation. Therefore, in-depth analysis of the spatial structure of lesion tissues, distribution of immune cell subsets, and bacterial colonization characteristics is crucial for revealing the pathogenesis of tuberculosis and evaluating the effectiveness of intervention measures.
Traditional research faces two major technical bottlenecks: first, conventional immunofluorescence techniques have low signal sensitivity and high background noise, making it difficult to detect low-abundance antigens; second, during multiplexed labeling, the limitation of antibodies from different species restricts the number of markers, and thin tissue sections cannot achieve complete characterization of 3D spatial structures. Additionally, the role of structural cells from pulmonary epithelial and mesenchymal lineages in the formation of Mtb-susceptible microenvironments has not been fully elucidated, and there is an urgent need for high-sensitivity imaging technology that can simultaneously preserve tissue integrity, endogenous fluorescent reporter signals, and nuclear staining. The optimized protocol established in the study by Lata S et al., titled "Protocol for 3D multiplexed fluorescent imaging of PTB lesions using Opal-TSA dyes for signal amplification," precisely addresses the above needs, achieving precise detection of multi-protein labeling in thick tissue sections and 3D reconstruction.
Core Methods

The core principle of TSA technology is that horseradish peroxidase (HRP)-conjugated secondary antibodies, in the presence of hydrogen peroxide, catalyze the conversion of fluorescently labeled tyramides into active forms, which then covalently bind to tyrosine residues near target antigens, achieving cascade amplification of signals. The study selected Opal fluorescent dyes as labels, which, compared to traditional fluorescent secondary antibodies, significantly improve signal sensitivity and allow imaging at low laser power, reducing tissue photobleaching.
The experimental cycle is approximately 3 days. The core workflow involves using Mtb-infected B6.Sst1S mice as a model to obtain lung tissues through perfusion fixation, followed by 4% agar embedding and vibrating microtomy, antigen retrieval through heat-induced treatment or Triton X-100 processing, blocking of non-specific sites with hydrogen peroxide and antibody diluent, then completing multiple labeling through cycles of primary antibody incubation, HRP-conjugated secondary antibody incubation, Opal dye incubation, antibody stripping, and washing, followed by nuclear staining. Finally, images are acquired using a confocal microscope with excitation and emission wavelengths matching the Opal dyes used for immunostaining, and 3D reconstruction is completed using Imaris 10.1.0 software.
Key Results
1. Enhanced Signal Sensitivity and Specificity, Reduced Background Noise
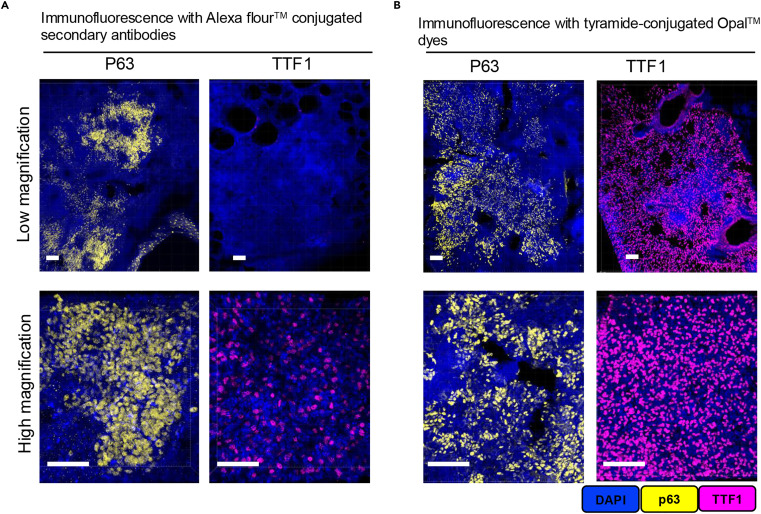
Compared to traditional immunofluorescence techniques using secondary antibodies conjugated with fluorophores, the signal sensitivity of Opal-TSA technology is significantly enhanced. The left panel shows imaging results of the low-abundance antigen TTF1 labeled with traditional Alexa Fluor 488, while the right panel shows the same antigen labeled with Opal 570. Both were captured using a 10× objective with identical exposure parameters. From the images, it can be clearly observed that the traditional technique group on the left only captures weak, diffuse fluorescence and cannot precisely localize antigen expression sites; the Opal-TSA group on the right presents bright, concentrated fluorescence signals that can accurately identify the distribution contours of TTF1-positive cells.
2. Enabling Multiplexed Labeling with Antibodies from the Same Species, Overcoming Labeling Quantity Limitations
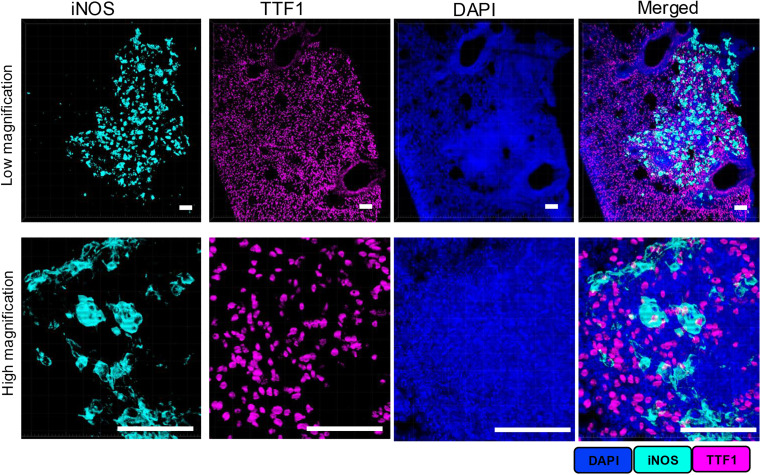
First, we focused on the feasibility of dual labeling with antibodies from the same species, using 50μm thick PTB lesion lung tissue sections as samples, validated through the "staining - stripping - restaining" workflow. Rabbit-derived iNOS-specific primary antibody was used for staining, developed with HRP-conjugated secondary antibody and Opal 690 dye; after stripping the primary/secondary antibody complex with mild BME/SDS stripping buffer, another rabbit-derived TTF1-specific primary antibody was added, paired with HRP secondary antibody and Opal 570 dye for secondary staining. Single-channel and merged images at both low and high magnification showed that the fluorescence signals of iNOS and TTF1 had clear boundaries without channel crosstalk, proving that the mild stripping protocol can completely remove the previous round of antibody complexes without affecting the already covalently bound Opal dye signals, directly validating the specificity and reliability of dual labeling with antibodies from the same species.
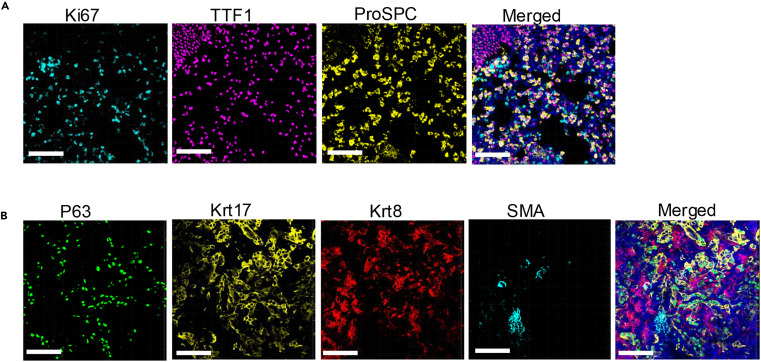
We further expanded to triple and quadruple labeling, with all primary antibodies being rabbit-derived and targets distinguished by Opal dyes of different spectra. The three fluorescence signals were localized to different cell populations in the lesion tissue without overlap interference; the four signals precisely corresponded to the expression characteristics of different cell types, with clear and distinguishable target distribution in merged images. Both sets of images demonstrated that this protocol, through optimized stripping and staining cycles, can achieve multiplexed labeling of at least 4 antibodies from the same species, solving the key bottleneck in traditional techniques where "antibody source limits labeling quantity," providing an efficient tool for multi-protein co-localization analysis in complex lesion tissues.
3. Preserving Tissue Integrity and Endogenous Signals, Supporting 3D Spatial Structure Analysis
Optimized sample preparation and processing workflows can completely preserve tissue integrity, endogenous fluorescent reporter signals, and nuclear staining, supporting 3D spatial structure analysis.
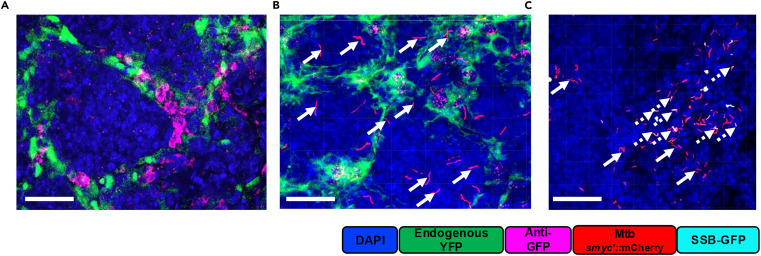
The above focuses on the compatibility of endogenous fluorescent reporter systems with Opal-TSA multiplexed immunofluorescence imaging. Using anti-GFP antibody staining, results showed that endogenous YFP reporter signals completely co-localized with antibody staining signals, proving that the protocol can preserve endogenous fluorescent protein activity without interfering with antibody detection. Merged imaging of anti-TTF1 antibody with bacterial reporter signals and YFP signals clearly presented the spatial distribution relationships of immune cells, bacteria, and pulmonary epithelial cells. Using B6.Sst1S mice without IFN β-YFP reporter genes as negative controls, only bacterial dual fluorescence signals were detected, with no non-specific YFP fluorescence, validating the specificity of endogenous signals.
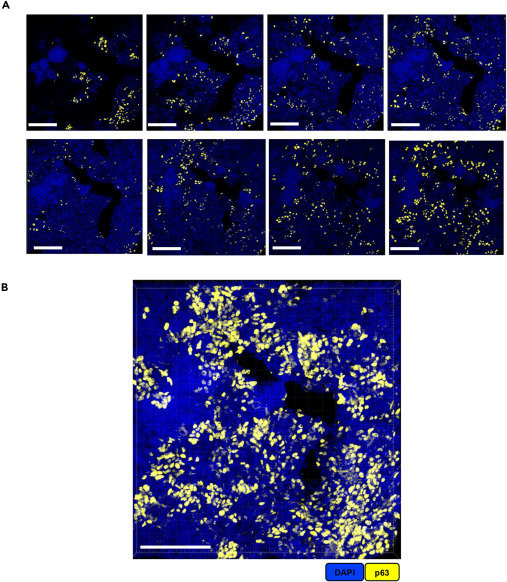
Using 50μm thick PTB lesion lung tissue sections as samples, with p63 antibody labeling targets, we present a comparison between 2D confocal images and 3D reconstructed images. A shows planar 2D images that can only display target distribution characteristics on a single plane; B shows 3D images reconstructed from the Z-stack sequence of this section using Imaris 10.1.0 software, intuitively presenting the three-dimensional clustered morphology of p63-positive cells in lesion tissues. Through direct comparison of the two imaging modes, the advantages of thick tissue sections combined with 3D reconstruction technology are highlighted, showing that compared to 2D planar imaging, it can more comprehensively and three-dimensionally reflect the spatial heterogeneity of lesion tissues.
4. Alternative Method to Traditional Heat-Induced Antigen Retrieval
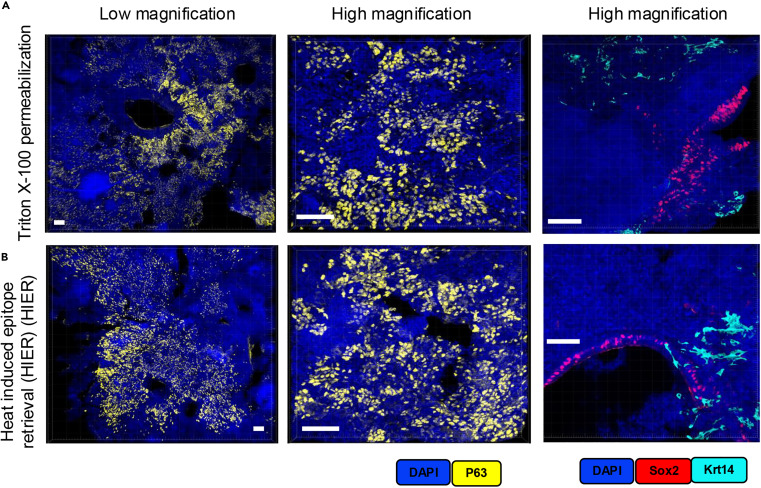
Using 50μm thick PTB lesion lung tissue sections as samples, we compared the cell permeabilization protocol of 2% Triton X-100 incubation at room temperature for 1 hour with traditional heat-induced antigen retrieval (HIER, Tris/borate/EDTA buffer incubation at 95°C for 30 minutes). Validation through p63 single-label imaging at low and high magnification, as well as Sox2 and Krt14 dual-label staining, showed that both protocols achieved precise antigen localization and specific detection. Moreover, the Triton X-100 group not only adapts to the multiplexed labeling workflow but also avoids tissue fragility and shrinkage issues caused by high temperatures in HIER. This demonstrates that the mild permeabilization protocol can achieve detection effects comparable to traditional HIER while better preserving thick tissue integrity, providing a more practical alternative for immunostaining of thick tissue sections.
Summary
This study systematically optimized Opal-TSA technology and established a 3D multiplexed fluorescent imaging protocol suitable for thick lung tissue sections from Mtb-infected mice. The core advantages include high signal sensitivity, multiplexed labeling capability with antibodies from the same species, preservation of tissue integrity and endogenous signals, and clear 3D spatial structure analysis. This protocol can precisely analyze the spatial distribution relationships between immune cells and structural cells in PTB lesions, as well as the survival and replication status of Mtb in lesions, providing a key technical tool for elucidating the role of pulmonary structural cells in the formation of Mtb-susceptible microenvironments and revealing the molecular mechanisms of tuberculosis progression. Additionally, the high sensitivity of the protocol allows rapid screening of regions of interest, improving the efficiency of subsequent high-resolution imaging and accelerating the discovery of intervention targets. This technology not only solves the key technical bottleneck in PTB lesion analysis but also provides an important reference for spatial characterization of tissues in other diseases.
References
Lata S, Yabaji SM, O'Connell AK, Gertje HP, Kirber MT, Crossland NA, Kramnik I. Protocol for 3D multiplexed fluorescent imaging of pulmonary TB lesions using Opal-TSA dyes for signal amplification. STAR Protoc. 2025 Mar 21;6(1):103640. doi: 10.1016/j.xpro.2025.103640. Epub 2025 Feb 20. PMID: 39982826; PMCID: PMC11889971.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
