Literature Sharing: Multiplex Immunofluorescence Analysis of Early Triple-Negative Breast Cancer
Background
Triple-negative breast cancer (TNBC) is the most aggressive subtype of breast cancer, with very poor prognosis and limited treatment options for advanced patients. Compared to other breast cancer subtypes, TNBC exhibits higher immunogenicity, with high enrichment of tumor-infiltrating lymphocytes (TILs) and high PD-L1 expression levels, making it a potential suitable population for immunotherapy. Previous studies have confirmed that stromal tumor-infiltrating lymphocytes (sTILs) have prognostic predictive value in TNBC and can predict the efficacy of neoadjuvant and adjuvant chemotherapy, but currently TILs have not been incorporated into routine clinical evaluation systems. The International Breast Cancer Study Group (IBCSG) 22-00 trial is a Phase III randomized clinical trial aimed at evaluating the efficacy of low-dose oral cyclophosphamide-methotrexate (CM) maintenance therapy in early hormone receptor-negative breast cancer patients after standard adjuvant chemotherapy. Previous studies found that CM maintenance therapy had no statistically significant effect on reducing relapse risk in TNBC patients, but patients with high sTILs levels (>500) showed more pronounced reduction in breast cancer relapse risk after receiving CM maintenance therapy. Based on this, Rusakiewicz S et al. characterized immune cell infiltration features in TNBC patients through 6-color immunofluorescence staining technology, evaluated the prognostic value of specific immune cell subsets and their predictive role in CM maintenance therapy efficacy, while analyzing the impact of immune cell spatial distribution characteristics on treatment efficacy and patient prognosis, providing evidence for the development of precision treatment strategies for TNBC patients.
Research Methods
The study cohort was derived from the intention-to-treat population of the IBCSG 22-00 trial, with confirmed TNBC status through central pathology review, and had formalin-fixed paraffin-embedded (FFPE) tissue sections and TILs detection results. FFPE tissue sections underwent HE staining and multiplex immunofluorescence (mIF) staining. HE-stained sections were reviewed by pathologists to confirm tissue tumor content and delineate regions of interest. mIF staining was performed after dewaxing, epitope retrieval, and endogenous peroxidase blocking, followed by sequential incubation with primary antibodies against CD4, FOXP3, CD3, cytokeratin, CD8, HRP-labeled secondary antibodies and specific fluorescent dyes, and finally stained with Spectral DAPI for nuclear staining and mounting, stored at 4°C in the dark and obtained multiplex IF images within 48h using a Vectra 3.0 imaging system. Tissue-specific and channel-specific spectral libraries for each fluorescent dye in each channel and tumor tissue autofluorescence were collected to achieve optimal immunofluorescence signal unmixing and multiplex analysis. After pre-scanning immunofluorescence-stained sections with 10× objective, Phenochart™ whole-section observation software was used to annotate regions of interest containing tumor islets and stroma, followed by 20× objective high-resolution multispectral image acquisition. Using inForm 2.3.0 software, tumor and stromal regions were distinguished based on cytokeratin staining, cell segmentation was performed using DAPI staining, and immune cell subsets were quantified through active learning phenotype algorithms, with cell population data ultimately exported and processed using self-developed R scripts. Additionally, based on CD3⁺ cell infiltration status in stroma and epithelium, tumors were classified into inflamed type (CD3⁺ cells infiltrating both stroma and tumor), excluded type (CD3⁺ cells mainly infiltrating stroma), and cold tumor (CD3⁺ cell infiltration very low) categories.
Association of Immune Cell Infiltration with Patient Prognosis
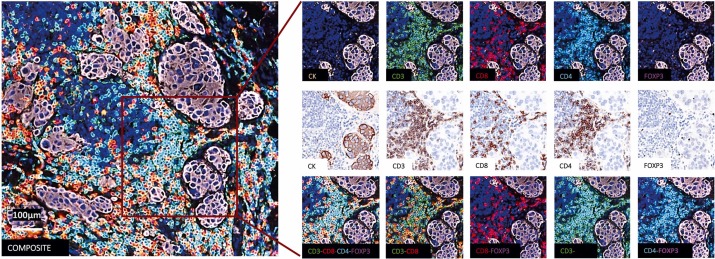
Multiplex immunofluorescence detection was performed on tumor tissue sections from 204 patients to observe immune cell infiltration in tumors. The experiment simultaneously marked 6 key markers: using CK to localize cancer cells, using CD3, CD4, CD8 to mark different types of T cells, using FoxP3 to mark regulatory T cells with immunosuppressive functions, and finally using DAPI to mark nuclei of all cells. The images clearly show the individual staining effects of each marker, corresponding tissue bright-field images, and combined staining results. From the detection results of this case, it was found that although the tumor sample had abundant immune infiltration, there was no immune cell infiltration into the CK-positive tumor cell nest area.
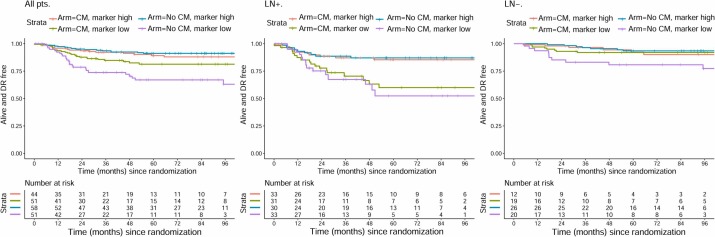
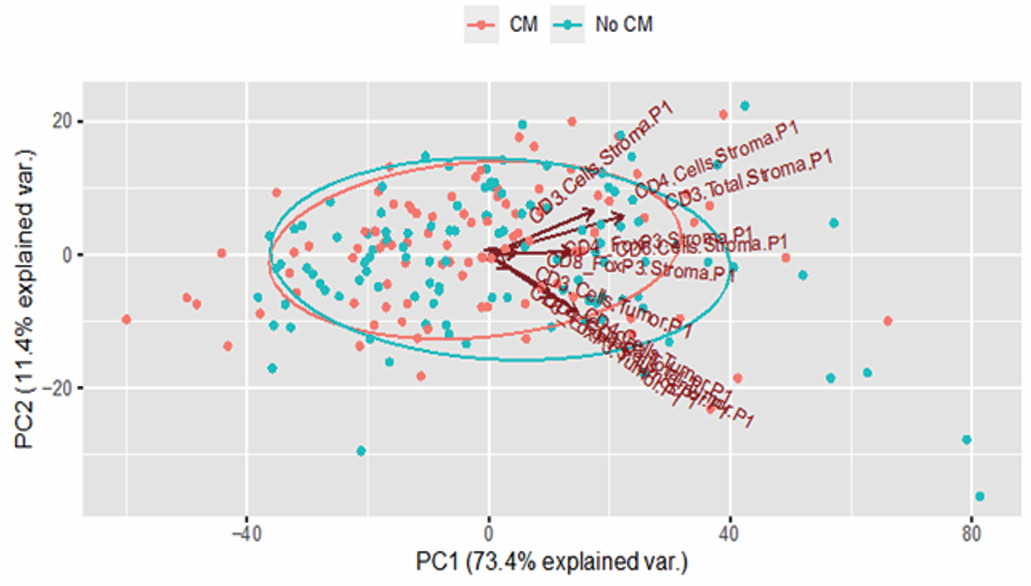
Principal component analysis was used for multivariate analysis of all immune cell infiltration data. Results showed that the first principal component could explain 73% of the variation in all detected markers, indicating that this dimension could well summarize the tumor's immune infiltration characteristics. From the overall patient data, the more immune cells in the tumor tissue, the lower the patient's risk of distant recurrence, and the longer the distant recurrence-free survival time. This trend was particularly evident in patients with lymph node metastases. However, there was also a contrary finding: for patients without lymph node metastasis and with very few immune cells in the tumor, their prognosis seemed to improve after CM maintenance therapy, with reduced relapse risk. But this result still needs validation with more patient data to confirm its reliability.
Association of Immune Cell Infiltration with CM Maintenance Therapy Efficacy
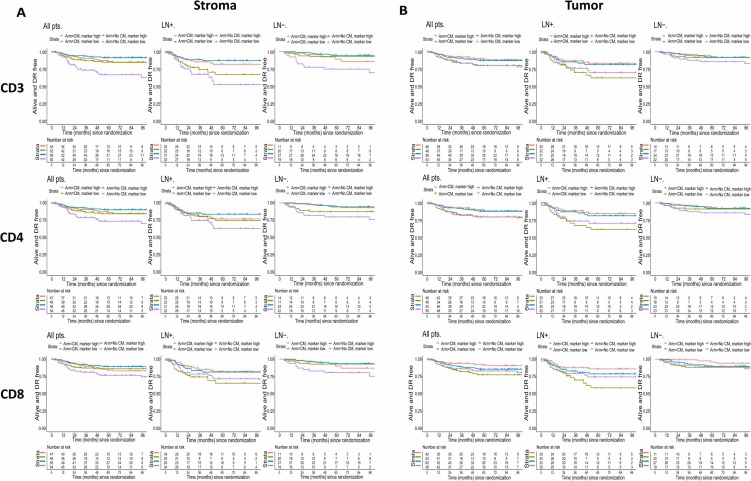
To clarify the impact of immune infiltration on patient distant recurrence-free interval (DRFI), the study conducted univariate analyses on the effects of CD3, CD4, CD8 and other T cell subsets, stromal tumor-infiltrating lymphocytes, and intraepithelial tumor-infiltrating lymphocytes. High stromal tumor-infiltrating lymphocyte levels were associated with better DRFI, particularly in LN + patients. Among these, the effect of CD3+ stromal tumor-infiltrating lymphocytes was more prominent in the group not receiving CM treatment, and the statistically significant interaction between this marker's dichotomous variables and treatment groups indicated that CM treatment might improve DRFI in the overall population with low stromal tumor-infiltrating lymphocyte levels and lymph node-negative patients. High intraepithelial tumor-infiltrating lymphocyte levels in tumor tissue had weaker improvement effects on DRFI, and were only evident in lymph node-positive tumor patients, while for patients with lymph node-positive disease and low intraepithelial CD8+ tumor-infiltrating lymphocyte levels, CM maintenance therapy might have adverse effects.
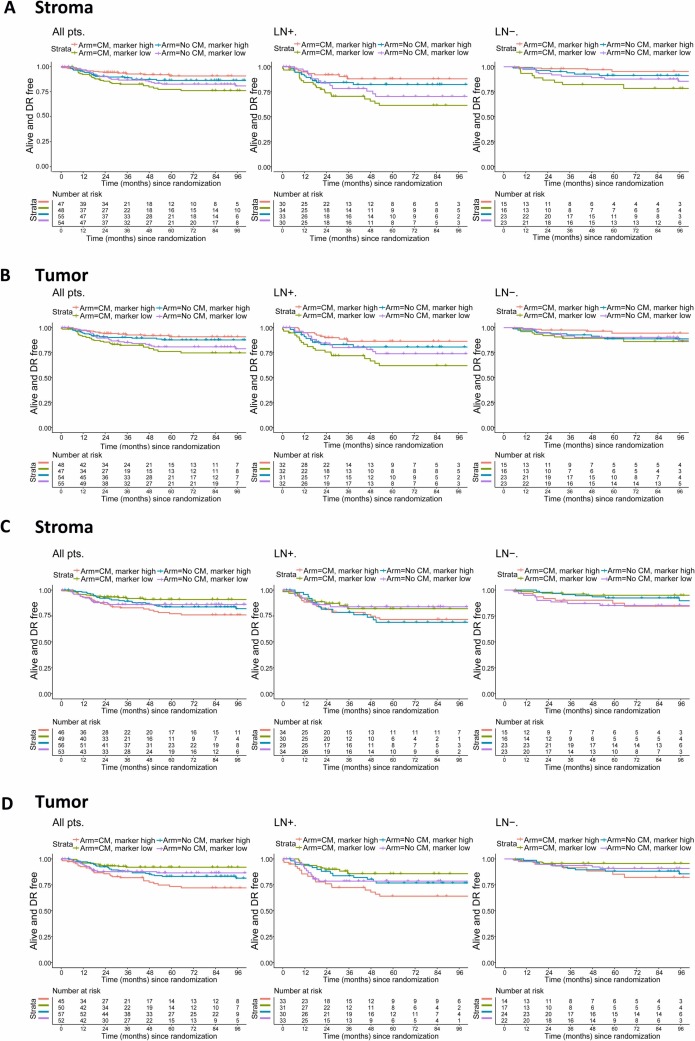
The study further combined treatment regimens and infiltration levels of stromal and intraepithelial regulatory T cells to assess patient DRFI, with related results shown above. In lymph node-positive patients, both high stromal regulatory T cell levels and high intraepithelial regulatory T cell levels were associated with longerDRFI, while patients with low stromal or intraepithelial regulatory T cell levels might experience adverse effects from CM maintenance therapy.
Impact of Immune Cell Spatial Distribution

Finally, the study evaluated the spatial distribution characteristics of immune cells in 204 triple-negative breast cancer samples and classified samples based on infiltration status of stromal CD3+ T cells and intraepithelial CD3+ T cells. Selected tumor regions of interest were first visualized, with Figure B showing typical examples of immune-inflamed, immune-excluded, and single immune cold tumor cases, while Figure C shows the distribution of CD3-excluded versus inflamed types among 203 triple-negative breast cancer samples. DRFI analysis based on this classification showed that CM maintenance therapy might have adverse effects on T cell-excluded lymph node-positive samples.
Conclusion
In this study on triple-negative breast cancer, multiplex immunofluorescence technology is the core technique for analyzing the tumor immune microenvironment and mining the associations between immune infiltration and prognosis as well as treatment efficacy. Through 6-marker combined staining, it precisely identifies and quantifies CD3⁺, CD4⁺, CD8⁺ T cells and regulatory T cells, clarifies the infiltration locations of these immune cells in tumor stroma and epithelium, and distinguishes stromal/intraepithelial tumor-infiltrating lymphocytes, providing foundational data for immune phenotyping. Additionally, the immune data obtained can reveal associations between high CD3⁺ T cell and regulatory T cell infiltration and DRFI, clarify the impact of different immune features on CM treatment efficacy, and provide immune phenotypic evidence for screening treatment-eligible populations. Furthermore, this technology can visualize immune cell spatial distribution through multispectral imaging, successfully classifying TNBC samples into inflamed, excluded, and cold tumor types, providing technical support for clinical stratification. It can also coordinate with HE staining to verify tissue content, combine automated tools to achieve precise single-cell quantification, avoid limitations of single markers, and provide high-quality data for statistical analysis.
References
Rusakiewicz S, Tyekucheva S, Tissot-Renaud S, Chaba K, Imbimbo M, Benedetti F, Kammler R, Hornfeld J, Munzone E, Gianni L, Thurlimann B, Láng I, Pruneri G, Gray KP, Regan MR, Loi S, Colleoni M, Viale G, Kandalaft L, Coukos G, Curigliano G. Multiplexed high-throughput immune cell imaging in patients with high-risk triple negative early breast cancer: Analysis from the International Breast Cancer Study Group (IBCSG) Trial 22-00. Eur J Cancer. 2024 Mar; 200:113535. doi: 10.1016/j.ejca.2024.113535. Epub 2024 Jan 24. PMID: 38309015.
Enkilife mIHC TSA Kits
Product | Catalog Number |
|---|---|
TSA Six-Label Seven-Color Multiplex Immunohistochemistry Kit | |
TSA Five-Label Six-Color Multiplex Immunohistochemistry Kit | |
TSA Four-Label Five-Color Multiplex Immunohistochemistry Kit | |
TSA Three-Label Four-Color Multiplex Immunohistochemistry Kit | |
TSA Two-Label Three-Color Multiplex Immunohistochemistry Kit |
