
Size:50μL Price:$118
Size:100μL Price:$220
Size:200μL Price:$380
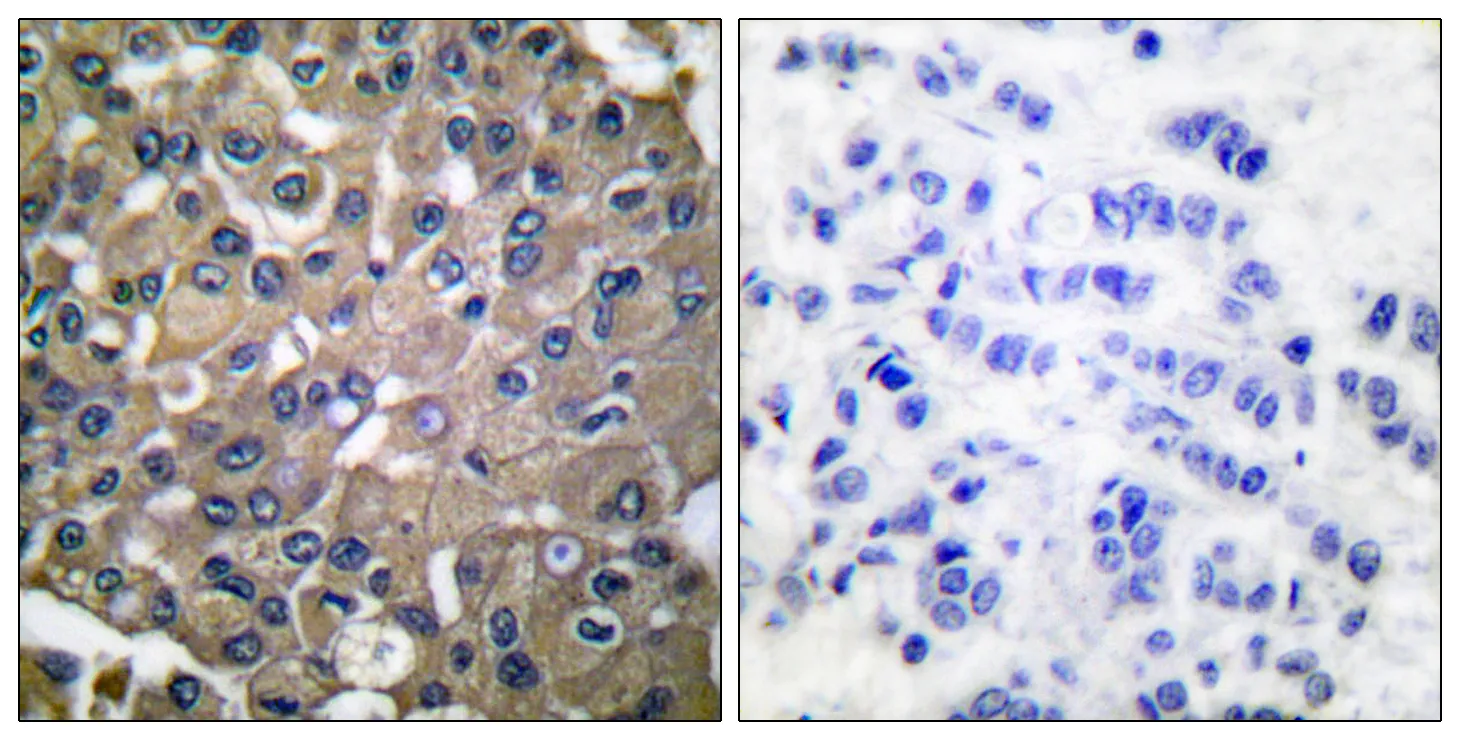
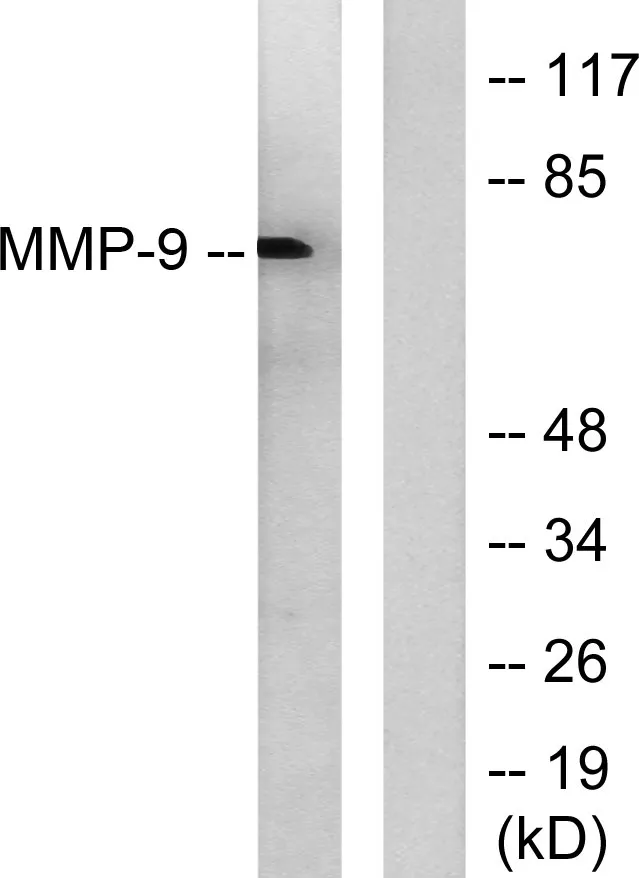
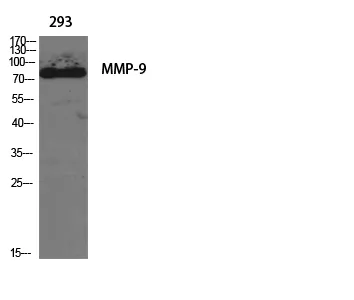
Application:WB,IHC,ICC/IF,ELISA
Reactivity:Human,Mouse,Rat
Conjugate:Unconjugated
Optional conjugates: Biotin, FITC (free of charge). See other 26 conjugates.
Gene Name:MMP9
Summary
Performance
Immunogen
Application
Background
matrix metallopeptidase 9(MMP9) Homo sapiens Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. The enzyme encoded by this gene degrades type IV and V collagens. Studies in rhesus monkeys suggest that the enzyme is involved in IL-8-induced mobilization of hematopoietic progenitor cells from bone marrow, and murine studies suggest a role in tumor-associated tissue remodeling. [provided by RefSeq, Jul 2008],catalytic activity:Cleavage of gelatin types I and V and collagen types IV and V.,cofactor:Binds 2 zinc ions per subunit.,cofactor:Binds 3 calcium ions per subunit.,disease:Defects in MMP9 may be a cause of susceptibility to lumbar disk herniation (LDH) [MIM:603932]. LDH is the predominant cause of low-back pain and unilateral leg pain.,domain:The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.,enzyme regulation:Inhibited by histatin-3 1/24 (histatin-5).,function:May play an essential role in local proteolysis of the extracellular matrix and in leukocyte migration. Could play a role in bone osteoclastic resorption. Cleaves KiSS1 at a Gly-|-Leu bond. Cleaves type IV and type V collagen into large C-terminal three quarter fragments and shorter N-terminal one quarter fragments. Degrades fibronectin but not laminin or Pz-peptide.,induction:Activated by 4-aminophenylmercuric acetate and phorbol ester.,miscellaneous:In the arthritis patient this enzyme might contribute to the pathogenesis of joint destruction and might constitute a useful marker of disease status.,PTM:N- and O-glycosylated.,PTM:Processing of the precursor yields different active forms of 64, 67 and 82 kDa. Sequentially processing by MMP3 yields the 82 kDa matrix metalloproteinase-9.,similarity:Belongs to the peptidase M10A family.,similarity:Contains 3 fibronectin type-II domains.,similarity:Contains 4 hemopexin-like domains.,subunit:Exists as monomer, disulfide-linked homodimer, and as a heterodimer with a 25 kDa protein. Macrophages and transformed cell lines produce only the monomeric form.,tissue specificity:Produced by normal alveolar macrophages and granulocytes.,
Research Area
Leukocyte transendothelial migration;Pathways in cancer;Bladder cancer;
