Size:100μL Price:$230
Size:200μL Price:$380
Application:WB,IHC,ICC/IF,FC
Reactivity:Human,Mouse,Rat
Conjugate:Unconjugated
Optional conjugates: Biotin, FITC (free of charge). See other 26 conjugates.
Gene Name:ATP5F1A
Summary
| Production Name | ATP5A1 (11Q15) Rabbit Monoclonal Antibody |
| Description | Recombinant rabbit monoclonal antibody |
| Host | Rabbit |
| Application | WB,IHC,ICC/IF,FC |
| Reactivity | Human,Mouse,Rat |
Performance
| Conjugation | Unconjugated |
| Modification | Unmodified |
| Isotype | IgG |
| Clonality | Monoclonal |
| Form | Liquid |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Buffer | Rabbit IgG in phosphate buffered saline , pH 7.4, 150mM NaCl, 0.02% New type preservative N and 50% glycerol. Store at +4°C short term. Store at -20°C long term. Avoid freeze / thaw cycle. |
| Purification | Affinity purification |
Immunogen
| Gene Name | ATP5F1A |
| Alternative Names | Atp5a1; ATP5AL2; ATPM; hATP1; HEL-S-123m; MC5DN4; MOM2; |
| Gene ID | 498 |
| SwissProt ID | P25705 |
Application
| Dilution Ratio | WB 1:1000-1:5000,IHC 1:50-1:200,ICC/IF 1:100-1:200,FC 1:20-1:50 |
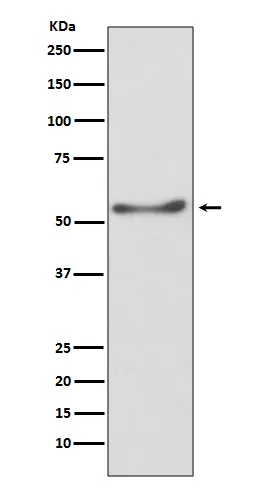
| Molecular Weight | 60kDa |
Background
Mitochondrial membrane ATP synthase (F(1)F(0) ATP synthase or Complex V) produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. F-type ATPases consist of two structural domains, F(1) - containing the extramembraneous catalytic core, and F(0) - containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. Mitochondrial membrane ATP synthase (F(1)F(0) ATP synthase or Complex V) produces ATP from ADP in the presence of a proton gradient across the membrane which is generated by electron transport complexes of the respiratory chain. F-type ATPases consist of two structural domains, F(1) - containing the extramembraneous catalytic core, and F(0) - containing the membrane proton channel, linked together by a central stalk and a peripheral stalk. During catalysis, ATP synthesis in the catalytic domain of F(1) is coupled via a rotary mechanism of the central stalk subunits to proton translocation. Subunits alpha and beta form the catalytic core in F(1). Rotation of the central stalk against the surrounding alpha(3)beta(3) subunits leads to hydrolysis of ATP in three separate catalytic sites on the beta subunits. Subunit alpha does not bear the catalytic high-affinity ATP-binding sites (By similarity). Binds the bacterial siderophore enterobactin and can promote mitochondrial accumulation of enterobactin-derived iron ions (PubMed:30146159).
Research Area
Mitochondria; ATPases; Mitochondrial markers; Complex V; Cancer
