Gastric adenocarcinoma is the most common type of pathology in gastric cancer (about 90% of all gastric cancers). The occurrence of gastric adenocarcinoma begins with the normal gastric mucosa and undergoes a gradual development process of "chronic inflammation→ atrophic gastritis→ intestinal metaplasia→ dysplasia (intraepithelial neoplasia)", each stage is accompanied by abnormal accumulation at the molecular level. The core mechanism can be divided into three major links: external triggering, chronic inflammatory drive, and molecular genetic abnormalities [1, 2].
External environmental factors are the core causes of gastric adenocarcinoma, among which Helicobacter pylori (Hp) infection is the most definitive class I carcinogenic factor (identified by WHO), and other factors exacerbate damage through synergistic or superimposing effects, such as long-term intake of a high-salt diet and genetic predisposition [3].
Mucosal barrier destruction and epithelial atrophy: inflammatory factors (such as IL-1β, TNF-α) inhibit the proliferation and differentiation of gastric mucosal epithelial cells, leading to fundic gland atrophy (decreased gastric acid secretion), further reducing the resistance of the mucosa to damaging factors;
Intestinal metaplasia (IM): In order to adapt to the environment of gastric acid reduction, the gastric mucosal epithelium is replaced by the intestinal epithelium (such as the appearance of goblet cells and absorbing cells), and this process is accompanied by changes in gene expression patterns (such as the expression of intestinal epithelial markers such as CDX2 and MUC2 is up-regulated), and the intestinal metaplasia epithelium has higher proliferative activity and is more prone to accumulation of mutations.
Dysplasia (intraepithelial neoplasia): Intestinal metaplasia further develops, epithelial cells have morphological and structural abnormalities (such as large nuclei, obvious nucleoli, and disordered arrangement), and cell proliferation is out of control (such as elevated Ki-67 index), but has not yet broken through the basement membrane (belongs to "precancerous lesions"), this stage is a key node in reversing cancer, and if not intervened, about 5%-10% of moderate to severe dysplasia will progress to adenocarcinoma within 5 years [4].
With the accumulation of mucosal damage, gastric mucosal cells gradually develop genetic mutations, chromosomal abnormalities, and epigenetic changes, ultimately leading to malignant phenotypes such as "unlimited proliferation, anti-apoptosis, and invasive metastasis".
RAS-MAPK pathway activation: Approximately 10%-20% of gastric adenocarcinomas have KRAS or NRAS gene mutations, leading to continuous activation of the pathway, promoting cell proliferation and inhibiting apoptosis.
PI3K-AKT-mTOR pathway activation: Approximately 30%-40% of gastric adenocarcinomas have PIK3CA gene mutations (encoding PI3K catalytic subunits) or PTEN gene inactivation (negatively regulating the PI3K pathway), which can promote cell metabolism, angiogenesis and invasion after pathway activation.
TP53 gene mutation: One of the most common mutations in gastric adenocarcinoma (incidence of about 50%-60%), TP53 is the "genome guardian" that loses the function of DNA damage repair and induce apoptosis after mutation, allowing abnormal cells to survive and accumulate more mutations;
Epstein-Barr virus (EBV) infection: Approximately 10% of gastric adenocarcinomas are associated with EBV infection (EBV-associated gastric cancer), which activates pathways such as NF-κB and PI3K by encoding proteins such as latent membrane protein 1 (LMP1), promoting inflammation and cell proliferation, while EBV infection can lead to abnormal DNA methylation (such as silencing tumor suppressor genes) [5].
DNA methylation: For example, the hypermethylation of the promoter region of tumor suppressor genes p16INK4a and MLH1 leads to gene silencing (unable to express proteins) and losing cell cycle regulation and DNA repair functions, respectively.
Histone modifications: such as histone deacetylate (HDAC) leads to chromatin concentration, and transcription of tumor suppressor genes is inhibited.
CAFs secrete ECM components such as collagen and fibronectin, forming a physical barrier that prevents immune cell infiltration. At the same time, it secretes cytokines such as IL-6 and TGF-β to promote the proliferation and invasion of tumor cells.
Immunosuppressive cells (such as Tregs) secrete IL-10, TGF-β, which inhibit effector T cell function, leading to "immune escape" and allowing tumor cells to grow.
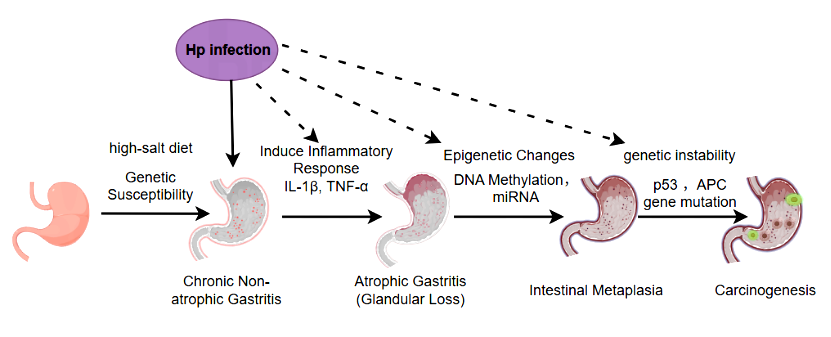
Diagram: Pathogenesis of Gastric Adenocarcinoma (Hp infection, high-salt diet, inflammatory response, genetic & epigenetic changes)
| Target | Catalog# | Product Name | Reactivity | Application |
|---|---|---|---|---|
| HP pathway-related antibodies | ||||
| COX-2 | AMRe09271 | COX2 (15D12) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, IP, IF-P |
| COX-2 | AMRe01845 | Cyclooxygenase 2 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P |
| Antibodies related to the uncontrolled pathway of gastric mucosal cell proliferation | ||||
| Cyclin D1 | AMRe09589 | Cyclin D1 (10Z18) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, IP, IF-P |
| p16INK4a | AMRe15577 | p16 INK (16J3) Rabbit Monoclonal Antibody | Human | WB, IHC-P, FC, IP, IF-P |
| p16INK4a | AMRe01811 | CDKN2A/p16INK4a Rabbit Monoclonal Antibody | Human, Mouse | WB, ICC/IF |
| CDKN1A | AMRe02380 | p21 Rabbit Monoclonal Antibody | Human, Mouse | WB |
| RB1 | AMRe03902 | Phospho-Rb (Ser807) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-F, IHC-P, ICC/IF |
| RB1 | AMRe05995 | Phospho-Retinoblastoma (S807) (4H3) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, IF-P |
| c-Myc | AMRe05879 | Phospho-c-Myc (S62) (9Z2) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| c-Myc | AMRe05880 | Phospho-c-Myc (T58) (1A2) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, ICC/IF, FC |
| HER2 | AMRe10568 | ErbB2 (HER2) (4J7) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| MET | AMRe02248 | c-Met Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-F, IHC-P, ICC/IF |
| CDK4 | AMRe01808 | CDK4 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-F, IHC-P, ICC/IF, IP |
| CDK6 | APRab08569 | Cdk6 Rabbit Polyclonal Antibody | - | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| Antibodies related to tumor microenvironment and angiogenesis pathway | ||||
| P53 | AMRe03901 | Phospho-p53 (Ser392) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-F, IHC-P, IP |
| P53 | AMRe02388 | p53 Rabbit Monoclonal antibody | Mouse | WB,ICC/IF,IP |
| Bcl-2 | AMRe03755 | Bcl2 Rabbit Monoclonal antibody | Human, Mouse | WB,IHC-P |
| Bax | AMRe03742 | Bax Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IP |
| Caspase-3 | AMRe01762 | Cleaved-Caspase 3 p12 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, ICC/IF |
| Caspase-3 | AMRe01567 | Caspase 3 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IP |
| Caspase-9 | AMRe04045 | Caspase 9 Rabbit Monoclonal Antibody | Human, Mouse | WB, IP |
| SMAD2 | AMRe03795 | Smad2 Rabbit Monoclonal antibody | Human,Rat,Hamster | WB,IHC-F,IHC-P,ICC/IF,IP |
| SMAD4 | AMRe03205 | Smad4 Rabbit Monoclonal Antibody | Human, Rat | WB, ICC/IF, IP |
| ZEB1 | AMRe20076 | ZEB1 (16B4) Rabbit Monoclonal Antibody | Human | WB, IHC-P, ICC/IF, FC, IF-P |
| AKT1 | AMRe06740 | AKT1 (5O1) Rabbit Monoclonal Antibody | Human, Mouse | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| PTEN | AMRe16636 | PTEN (16Q18) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, FC, IP, IF-P |
| mTOR | AMRe02286 | Phospho-mTOR (Ser2448) Rabbit Monoclonal Antibody | Human, Mouse | WB, IHC-P |
| KRAS | APRab13128 | K-Ras Rabbit Polyclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| NRAS | AMRe02525 | GTPase HRAS Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, ICC/IF, IP |
| ERK1 | AMRe03741 | ERK1/2 Rabbit Monoclonal antibody | Human, Mouse, Rat | WB,ICC/IF,IP |
| VEGFA | AMRe02757 | VEGFA Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB |
| FGFR2 | AMRe10945 | FGFR2 (18K11) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IP |
| CA9 | AMRe07799 | CA9 (14N17) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IP, IF-P |
| CLDN1 | AMRe08890 | Claudin 1 (5F6) Rabbit Monoclonal Antibody | Human | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| Antibodies associated with inflammatory and immune regulatory pathways | ||||
| TNFα | AMM19084 | TNF α(Q34) Mouse Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IF-P, IF-F, ICC/IF |
| IL-6 | APRab03851 | IL-6 Rabbit Polyclonal Antibody | Human | WB, IHC-P, ELISA |
| IL-8 | AMRe12568 | IL8 (6Z6) Rabbit Monoclonal Antibody | Human | WB |
| IL-10 | AMRe12483 | IL10 (8U9) Rabbit Monoclonal Antibody | Human | WB, ICC/IF, FC |
| TGF-β1 | AMM00661 | TGF beta 1 (8F6) Mouse Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P |
| STAT3 | AMRe06021 | Phospho-STAT3 (Y705) (13H8) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| STAT3 | AMRe18352 | STAT3 (11W6) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, ICC/IF, FC, IF-P |
| Galectin-9 | APRab11278 | Galectin-9 Rabbit Polyclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| NLRP3 | AMRe01571 | NLRP3 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB |
| NLRP3 | AMRe14399 | NALP3 (8Q17) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, FC, IP |
| PD-L1 | AMRe15922 | PD-L1 (CD274) (5R18) Rabbit Monoclonal Antibody | Human | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| PD-1 | AMRe15873 | PD L2 (12P7) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB |
| CTLA-4 | AMRe09507 | CTLA4 (CD152) (14H2) Rabbit Monoclonal Antibody | Human, Mouse | WB, IHC-P, FC, IP, IF-P |
| Other relevant antibodies | ||||
| Vimentin | AMRe03745 | Vimentin Rabbit Monoclonal Antibody | Human, Mouse, Rat, Hamster | WB, IHC-F, IHC-P, ICC/IF |
| CD44 | AMRe08400 | CD44 (19J7) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IP, IF-P |
| E-Cadherin | AMRe01411 | E Cadherin Rabbit Monoclonal Antibody | Human | WB,IHC-F,IHC-P,ICC/IF,IP |
| MMP3 | AMRe02266 | MMP3 Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC-P |
| MMP7 | APRab13996 | MMP-7 Rabbit Polyclonal Antibody | Human, Mouse, Rat, Monkey | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| MMP9 | AMRe02267 | MMP9 Rabbit Monoclonal Antibody | Rat | WB, IHC-P, IP |
| MMP9 | APRab14000 | MMP-9 Rabbit Polyclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| MMP13 | APRab13979 | MMP-13 Rabbit Polyclonal Antibody | Human, Rat, Mouse | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| CA125 | APRab14240 | Mucin 16 Rabbit Polyclonal Antibody | Human, Rat, Mouse | WB, IHC-P |
| Target | Catalog# | Product Name | Reactivity | Detection Range | Sensitivity |
|---|---|---|---|---|---|
| VEGFA | EM10657 | Mouse VEGF-A (Vascular Endothelial Cell Growth Factor A) ELISA Kit | Mouse | 31.25-2000pg/mL | 18.75pg/mL |
| MMP3 | EH10202 | Human MMP-3 (Matrix Metalloproteinase 3) ELISA Kit | Human | 0.16-10ng/mL | 0.1ng/mL |
| MMP9 | EM10682 | Mouse Pro-MMP-9 (Pro-Matrix Metalloproteinase-9) ELISA Kit | Mouse | 78.13-5000pg/mL | 46.88pg/mL |
| MMP9 | EH10079 | Human MMP-9 (Matrix Metalloproteinase 9) ELISA Kit | Human | 0.16-10ng/mL | 0.1ng/mL |
| CA125 | EH10414 | Human CA125 (Carbohydrate Antigen 125) ELISA Kit | Human | 3.13-200IU/mL | 1.88IU/mL |
| TNFα | EH10021 | Human TNF-α (Tumor Necrosis Factor Alpha) ELISA Kit | Human | 7.81-500pg/mL | 4.69pg/mL |
| TNFα | EM27661S | High Sensitivity Mouse TNF-α (Tumor Necrosis Factor Alpha) ELISA Kit | Mouse | 1.56-100pg/mL | 0.93pg/mL |
| IL-1β | EM27654S | Mouse IL-1β (Interleukin 1 Beta) ELISA Kit | Mouse | 3.13-200pg/mL | 1.87pg/mL |
| IL-6 | EM21023S | High Sensitivity Mouse IL-6 (Interleukin 6) ELISA Kit | Mouse | 0.781-50pg/mL | 0.47pg/mL |
| IL-6 | EH10020 | Human IL-6 (Interleukin 6) ELISA Kit | Human | 1.56-100pg/mL | 0.94pg/mL |
Related Products
Wang R, Song S, Harada K, et al. Multiplex profiling of peritoneal metastases from gastric adenocarcinoma identified novel targets and molecular subtypes that predict treatment response. Gut. 2020 Jan;69(1):18-31. Epub 2019 Jun 6. [PMID: 31171626].
Song S, Fan Y, Zou G, Huo L,et al. KAP1 promotes gastric adenocarcinoma progression by activating Hippo/YAP1 signaling via binding to HNRNPAB. Cancer Lett. 2025 Jul 1;621:217695. Epub 2025 Apr 4. [PMID: 40189014].
Ng D, Cyr D, Khan S, Dossa F, Swallow C, Kazazian K. Molecular mechanisms of metastatic peritoneal dissemination in gastric adenocarcinoma. Cancer Metastasis Rev. 2025 May 3;44(2):50. [PMID: 40317360].
Wiklund AK, Santoni G, Yan J, Radkiewicz C, et al. Risk of Gastric Adenocarcinoma After Eradication of Helicobacter pylori. Gastroenterology. 2025 Aug;169(2):244-250.e1. Epub 2025 Feb 7. [PMID: 39924057].
Song D, Liu Q, Yan Z, Wang Q, et al. Inhibition of gastric adenocarcinoma proliferation by WSGC@MS: Role of KEAP1/NRF2 signaling pathway and autophagy regulation. Mater Today Bio. 2025 Jun 16;33:101995.[ PMID: 40688680].
 | Flora Flora is a technical support expert at EnkiLife, familiar with immunology and neuroscience, dedicated to providing customers with high-quality product combinations and technical support to help achieve research in neurodegenerative diseases and other neuroscience areas. |
© 2025 EnkiLife Gastric adenocarcinoma Research Materials | Providing Professional Antibodies and ELISA Kits
