Common Antibody Purification Methods
Antibody purification refers to the process of isolating polyclonal antibodies from serum, or monoclonal antibodies from ascites fluid or the culture supernatant of hybridoma cell lines. Its core goal is to obtain antibodies with high purity, high activity, and that meet the requirements of specific applications.
Antibody purification is a process that relies on the biological characteristics of antibodies—such as the charge, size, and hydrophobicity of antibody molecules—to select appropriate methods and isolate target antibodies from complex mixtures through multi-step chromatographic techniques.
Generally, antibody purification methods include Protein A/G affinity chromatography, ion exchange chromatography, gel filtration chromatography, hydrophobic interaction chromatography, and others, which ensure the final acquisition of high-purity antibody products. A single, rapid capture step using affinity chromatography is sufficient to achieve the required purity and quantity of the target product. However, antibodies or their fragments can be further purified for subsequent use by removing more excess impurities through other methods.
The selection of a specific purification method depends on factors such as the source of the antibody, time constraints, cost considerations, and the final application of the antibody.
Caprylic Acid-Ammonium Sulfate Precipitation Method
Caprylic acid-ammonium sulfate precipitation method by leveraging the properties of caprylic acid and ammonium sulfate, the caprylic acid-ammonium sulfate method selectively precipitates and purifies antibodies, and is primarily used to extract IgG antibodies from serum or ascites fluid.
Under slightly acidic conditions, caprylic acid binds to and precipitates other proteins in serum or ascites fluid, while IgG antibodies remain in the supernatant. This is because caprylic acid disrupts the hydration shells of other proteins, causing them to precipitate, whereas IgG antibodies—due to their structural characteristics—are less prone to precipitation.
After treatment with caprylic acid, ammonium sulfate saturation is used for salting out. Ammonium sulfate competes with antibodies for water molecules in the solution, reducing the solubility of antibodies and thereby causing them to precipitate. An equal volume of saturated ammonium sulfate solution is slowly added to the neutralized antibody sample, followed by incubation at room temperature or 4°C for several hours. After centrifugation and removal of the supernatant, the antibody precipitate is dissolved in a buffer solution.
The selectivity, yield, purity, and reproducibility of precipitation depend on several factors, including time, temperature, pH, and the rate of salt addition. Ammonium sulfate precipitation can provide sufficient purification efficacy for certain antibody applications; however, in most cases, it serves as a preliminary purification step prior to column chromatography or other purification methods.
Affinity Chromatography Method
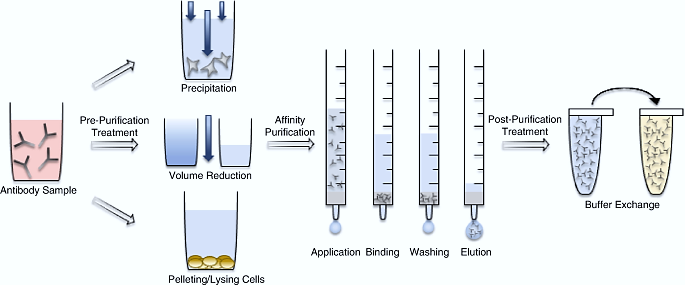
Antibody Purification Using Affinity Chromatography[1]
Affinity chromatography is a highly efficient separation and purification technology based on the specific recognition of biomolecules. This method forms a separation system with selective adsorption capacity by conjugating specific ligands (such as Protein A/G/L) to solid-phase matrices like agarose. When a complex mixture is added, the ligand can specifically capture the target antibody, while impurities are eluted, and the target antibody is finally obtained. This technology is particularly suitable for isolating antibodies from mixtures with complex components, where the content of impurities is much higher than that of the target antibody.
Protein A can efficiently bind IgG from various mammals, including humans, mice, rabbits, pigs, and dogs. Among them, it exhibits extremely high affinity for human IgG1, IgG2, and IgG4 subtypes, but has weak binding to IgG3.
Compared with Protein A, Protein G has higher binding affinity for IgG from most mammals and can specifically bind to a wider range of antibody types—especially those that are difficult for Protein A to bind, such as human IgG3, mouse IgG1, and rat IgG2a. Recombinant Protein G has its albumin-binding sites and cell surface-binding sites removed, which not only significantly reduces non-specific binding but also improves the purity of the product. This protein has become an important tool for antibody purification, and is particularly suitable for purifying mammalian monoclonal and polyclonal IgG antibodies that cannot bind well to Protein A.
Protein L can specifically bind to κ light chains through its five N-terminal homologous domains. This binding mode enables it to capture various antibody molecules containing κ light chains. The protein can effectively bind to multiple antibody types, such as IgG, IgM, IgA, IgE, and IgD, and can also recognize antibody derivatives like single-chain variable fragments (ScFv) and fragment antigen-binding (Fab) fragments. Protein L has good binding ability to antibodies derived from humans, mice, rats, and rabbits, but has no binding activity to immunoglobulins from goats and sheep. Protein L provides an important solution for the purification of antibodies with special structures.
Overview of bioaffinity ligands Protein-A, -G, -L, and -M[2]
| Protein Type | Binds to | Advantages | Disadvantages |
|---|---|---|---|
| Protein-A | •IgG, IgG fragments and subclasses (except IgG₃), hinge region connecting Cₕ₂ and Cₕ₃ on the Fc region of IgG (high affinity ~ 10 nM) •Fc fusion proteins •IgM and IgE | •High flow rate •High binding capacity •Binds Fc and Fab and F(ab’)₂ fragments of IgG | •Some nonspecific binding of host cell proteins |
| Protein-G | •IgG₁, IgG₂, IgG₃, and IgG₄ •Hinge region connecting Cₕ₂ and Cₕ₃ on the Fc region of IgG (high affinity ~10 nM) •Constant domain of the Fab through Cₕ₁ (low affinity μΜ) •Human IgE, IgD, and IgA | •Wider reactivity profile to IgG than Protein-A | •Does not withstand as harsh elution conditions as Protein-A |
| Protein-L | •Vₗ of kappa light chains (Kₚ ~ 100 mM), binds to IgG, IgM, IgA, IgE, and IgD (containing variable light chains) | •Very effective at binding antibodies in ascites fluid or cell culture supernatant •Very effective binding of IgM | •Not useful for purifying polyclonal antibodies |
| Protein-M | •Protein M binds to the conserved regions (framework regions) of the Vₗ and Vₖ antibody light chains | •Broad antibody specificity | •Not yet fully investigated |
Antigen Affinity Purification Method
Antigen affinity purification is a highly efficient antibody purification technology based on the principle of antigen-antibody specific recognition. In this method, the target antigen is used as an affinity ligand and immobilized on a gel matrix through chemical conjugation to form a highly selective affinity chromatography system. The target antibody specifically binds to the antigen, and finally, the target antibody is obtained through elution.
Different from traditional Fc region-binding methods such as Protein A/G, antigen affinity purification directly targets the variable region of antibodies, enabling precise capture of antibodies specific to a particular antigen epitope.
The key advantage of the antigen affinity purification method lies in its high specificity, making it particularly suitable for isolating antigen-specific antibodies from polyclonal antibody mixtures. Since a large amount of high-purity antigen is required as the ligand, this method usually necessitates pre-synthesis or purification of the target antigen. Compared with conventional Fc region-binding purification methods, antigen affinity purification exhibits higher selectivity.
Antibodies purified by this method, characterized by high sensitivity and specificity, are suitable for immunological detection experiments such as Immunohistochemistry (IHC) and Immunofluorescence (IF). Even when used at a relatively low dilution ratio, they do not increase the signal background.
Ion Exchange Chromatography Method
Ion exchange chromatography relies on the net charge of proteins in a specific buffer system, using resins with positive or negative charges to bind to proteins. Antibodies are proteins composed of amphoteric amino acids, which contain side chains with acidic or basic functional groups that can ionize into ions in a buffer solution of a certain pH.
The isoelectric point (pI) is generally used as a standard for selecting experimental conditions in ion exchange chromatography. Typically, it is divided into two types—anion exchange chromatography and cation exchange chromatography—based on the properties of the ion exchange stationary phase.
When antibodies are in a pH environment lower than their isoelectric point, they mainly undergo basic dissociation, exist in a cationic state with a positive charge, and can bind to cation exchangers. In contrast, when antibodies are in a pH environment higher than their isoelectric point, they mainly undergo acidic dissociation, exist in an anionic state with a negative charge, and can bind to anion exchangers.
Substances to be separated have different affinities for the immobilized ion exchange groups; therefore, the purpose of separating antibodies can be achieved by adjusting the ionic strength and pH value of the buffer solution.
Hydrophobic Interaction Chromatography Method
Hydrophobic Interaction Chromatography (HIC) is a chromatographic technique that achieves separation based on differences in hydrophobic forces between the hydrophobic groups of sample molecules and the hydrophobic ligands of the chromatographic medium in a salt-water system.
Monoclonal antibodies contain numerous non-polar amino acids in their molecular structure. The side chains of these non-polar amino acids exposed on the surface of antibody molecules can cluster together to form hydrophobic regions, which play a role in stabilizing the tertiary and quaternary structures of the antibody. There are significant differences in the strength of hydrophobic regions among different monoclonal antibodies.
As the salt concentration in the solution increases, hydrophobic interactions become stronger. Therefore, most hydrophobic interaction chromatography processes involve loading samples under high-salt concentration conditions and performing elution under low-salt concentration conditions.
An important characteristic of hydrophobic interaction media is their weak hydrophobicity; their interaction with proteins is relatively mild. This enables them to better preserve the natural structure and biological activity of biomacromolecules. Meanwhile, hydrophobic interaction chromatography is also highly effective in extracting target proteins with low content from large-volume samples.
Gel Filtration Chromatography Method
Gel filtration chromatography, also known as size exclusion chromatography or molecular sieve chromatography, is a key technology for separation based on differences in molecular size during antibody purification.
This method uses porous gel particles as the stationary phase, allowing molecules in the solution to be separated in order of decreasing size in the solution. When a mixed sample containing antibodies flows through the chromatographic column, antibodies with larger molecular weights cannot enter the pores of the gel and are thus eluted rapidly. In contrast, small-molecule impurities penetrate deep into the pores, resulting in a slow flow rate and delayed elution—thereby achieving efficient separation.
Although the resolution of this method is lower than that of some specific purification techniques, it has advantages such as simple operation, mild conditions, and a wide range of applications.
Recombinant rabbit monoclonal antibodies launched by EnkiLife provide efficient solutions for scientific research needs, leveraging an extensive portfolio of over 7,000 varieties and full compatibility with diverse experimental scenarios.Visit our website to view Recombinant Rabbit Monoclonal Antibody.
References
[1] Cassedy, A., O’Kennedy, R. (2022). Antibody Purification Using Affinity Chromatography. In: Ayyar, B.V., Arora, S. (eds) Affinity Chromatography. Methods in Molecular Biology, vol 2466. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2176-9_1.
[2] Murphy, C., Devine, T., & O’Kennedy, R. (2016). Technology advancements in antibody purification. Antibody Technology Journal, 6, 17–32. https://doi.org/10.2147/ANTI.S64762.
 | Felicia Felicia is a technical support specialist at EnkiLife, with extensive professional experience in antibody development, optimization, and ELISA assay design and application. She is committed to assisting our clients in selecting suitable antibody products, optimizing ELISA experimental protocols, and resolving technical challenges encountered in the process, thereby supporting the smooth progress of their life science research projects. |
