Antibody Isotype and Antibody Subtype
Antibodies, known as immunoglobulins, are typically "Y"-shaped. In most mammals, immunoglobulins consist of five types: IgG, IgM, IgA, IgD, and IgE, which differ from one another in size, charge, amino acid composition, and carbohydrate content. Meanwhile, each type of antibody has subclasses with heterogeneity among them; for instance, the four subtypes of IgG (IgG1, IgG2, IgG3, IgG4) in humans and mice do not share homology. Besides, IgY antibodies are also present in avian species.
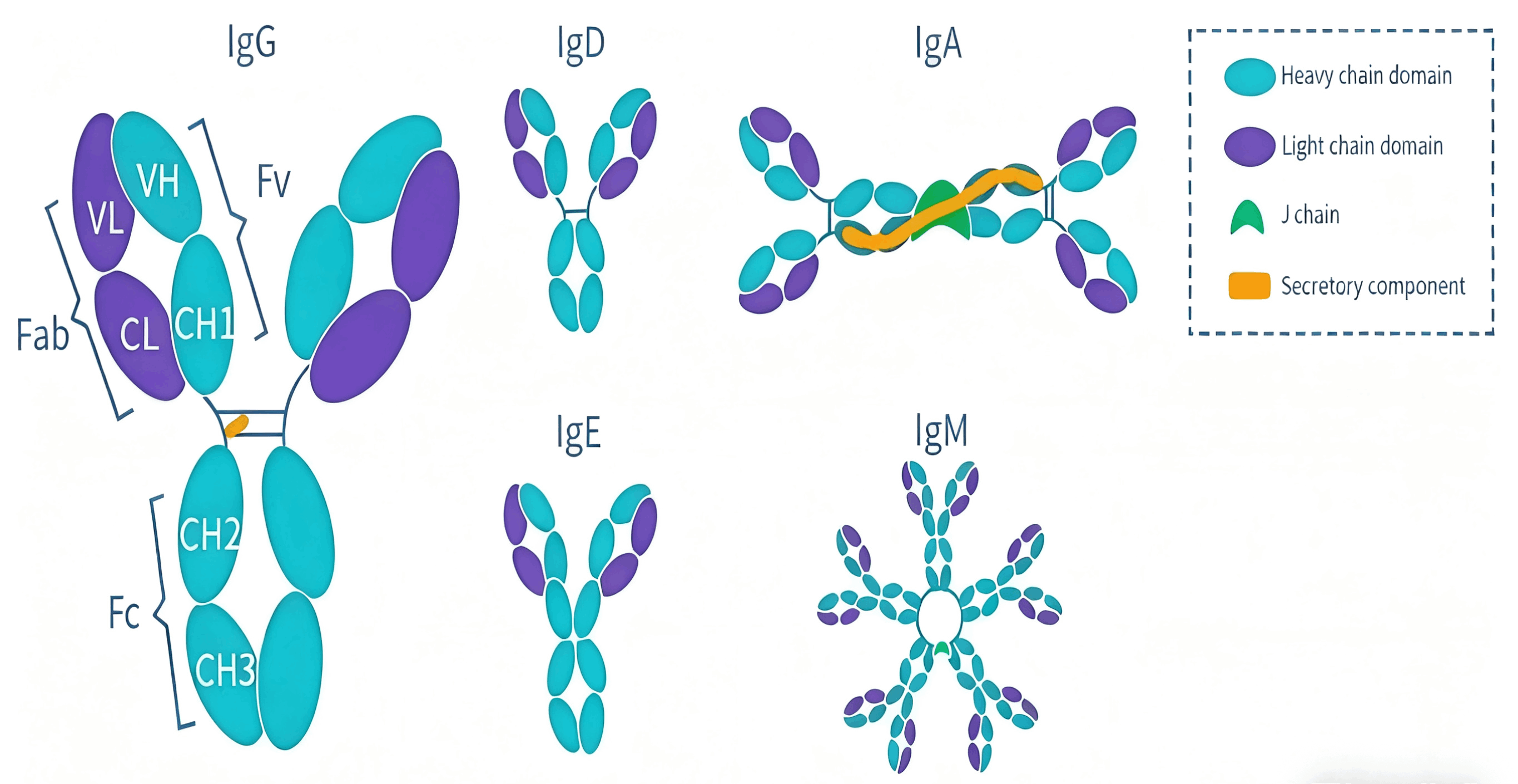
Antibody Isotype
IgG
IgG is present in the blood and extracellular fluid and is the most predominant antibody in the body, accounting for 70-85% of the total immunoglobulin population. It has a molecular weight of approximately 150 kD and usually exists in a monomeric form. Based on structural differences in constant region genes and the ability to trigger distinct effector functions, IgG is classified into 4 subclasses, including IgG1, IgG2, IgG3, and IgG4. These subclasses share a high degree of sequence similarity (approximately 90% identity) but exhibit differences in half-life, antigen-binding characteristics, and complement activation capacity. IgG possesses antiviral, virus-neutralizing, antibacterial, and immunomodulatory functions. It is also the only antibody capable of crossing the placenta, playing a crucial role in protecting newborns against infections.
In immunoassays, IgG is almost exclusively used, owing to the following advantages:
It has the highest yield in immune responses.
It exhibits the strongest binding affinity to antigenic epitopes.
It maintains stability during isolation and purification processes.
It contains multiple functional sites that can be used for chemical conjugation with minimal loss of antibody binding capacity.
IgA
IgA is the second most abundant antibody in the human immune system. Its core function is mucosal immune defense, while it also participates in humoral immune regulation. It exists in two forms: serum IgA and secretory IgA (sIgA). Serum IgA is mostly monomeric and participates in systemic humoral immunity. Secretory IgA is dimeric, serving as the primary antibody on mucosal surfaces, and is present in saliva, tears, breast milk, as well as secretions of the respiratory tract, digestive tract, and reproductive tract. IgA is divided into two subtypes, IgA1 and IgA2, which differ in the size of their hinge regions. IgA1 has a longer hinge region, which increases its susceptibility to bacterial proteases. For this reason, this subtype dominates serum IgA, whereas IgA2 is mainly found in mucosal secretions.
IgM
IgM is the first immunoglobulin expressed during B cell development and the primary antibody in the primary immune response, with a molecular weight of approximately 900 kDa. It can exist in either monomeric or pentameric forms: the monomeric form binds to the surface of B cells, while the secretory form is a pentamer linked by disulfide bonds and a J chain. The pentameric structure enables IgM to bind to antigenic epitopes efficiently. However, it is only of limited use in assays (such as immunohistochemistry) that require signal amplification, and may exhibit the effect of macromolecular steric hindrance. Moreover, compared with IgG, IgM is mainly produced in the early stages of the immune response, has lower affinity, and is more difficult to purify and isolate.
IgD
IgD is present in very low levels in the serum, accounting for less than 1% of the immunoglobulin pool, with a molecular weight of 180 kDa. It mainly exists as a membrane receptor on the surface of B cells. The expression level of the IgD isotype is associated with the activation state of B cells. Currently, there is relatively little research on IgD, and people have limited knowledge about its role in the serum.
IgE
IgE is an antibody with the lowest content but unique functions in the human immune system, with a molecular weight of approximately 190 kDa. Most IgE molecules bind to the FcεRI receptors on the surface of mast cells and basophils through their Fc regions, remaining attached to the cell surface for an extended period. Similar to the structure of IgM, IgE lacks a hinge region but has two additional constant domains that replace the hinge region. The core function of IgE antibodies is to mediate type I hypersensitivity reactions, while also participating in anti-parasitic immunity.
IgY
IgY is an antibody unique to avian, reptilian, and amphibian species. In terms of function, structure, and size, it is similar to IgG. It mainly exists in a monomeric form, with only a secretory type. Primarily present in avian serum and egg yolk, its core function is humoral immune defense, and it also possesses unique scientific research and application value.
Antibody Subtype
Based on differences in hinge region size, the position of interchain disulfide bonds, and molecular weight, IgG in humans and mice can be further subdivided into four subtypes. Human IgG is classified into IgG1, IgG2, IgG3, and IgG4, which are named in the order of their abundance in serum. Mouse IgG is categorized into IgG1, IgG2a, IgG2b, IgG2c and IgG3. After the preparation of hybridoma monoclonal antibodies is completed, it is necessary to identify the antibody subtype.
Human IgG
IgG1 accounts for approximately 60-65% of the total IgG content. It is primarily responsible for thymus-mediated immune responses against protein and peptide antigens. It also participates in the opsonization and activation of the complement cascade. A deficiency in the IgG1 isotype is often a sign of hypogammaglobulinemia.
IgG2 makes up around 20-25% of the total IgG content. It mainly mediates immune responses against carbohydrate/polysaccharide antigens. Among all IgG isotype deficiencies, IgG2 deficiency is the most common cause of recurrent airway/respiratory tract infections in infants.
IgG3 constitutes 5-10% of the total IgG content and plays a major role in immune responses against protein or peptide antigens.
IgG4 typically accounts for less than 4% of the total IgG content and does not bind to polysaccharides. Recent studies have shown that elevated serum IgG4 levels are detected in patients with sclerosing pancreatitis, cholangitis, and interstitial pneumonia. Currently, other functions of IgG4 remain unknown.
Mouse IgG
IgG1 is closely associated with humoral immunity and can effectively neutralize antigens, primarily functioning in responding to foreign antigens.
IgG2a exhibits a strong proinflammatory effect, often activating the complement system to participate in immune defense, and is an important target in research on infection and anti-tumor immunity.
IgG2b plays a role in immune regulation and inflammatory balance, and is commonly used to study the regulatory mechanisms of immune responses.
IgG2c exists only in certain mouse strains (e.g., C57BL/6), has functions similar to IgG2a, and plays a significant role in immune responses.
IgG3 possesses multivalent binding capacity but has a low natural distribution, and is frequently used in studies related to complement reactions.
Different types of antibodies exhibit differences in structure and function, which determine their distinct roles in the body. IgG products are widely used in immunological research, diagnosis, and therapy. They are commonly applied in experiments such as antibody identification, immune response analysis, vaccine development, and cell labeling and sorting. EnkiLife offers over 7,000 types of recombinant antibodies, covering various antibody categories. For more details, please visit the official website if needed. Visit our website to view Recombinant Rabbit Monoclonal Antibody.
 | Felicia Felicia is a technical support specialist at EnkiLife, with extensive professional experience in antibody development, optimization, and ELISA assay design and application. She is committed to assisting our clients in selecting suitable antibody products, optimizing ELISA experimental protocols, and resolving technical challenges encountered in the process, thereby supporting the smooth progress of their life science research projects. |
