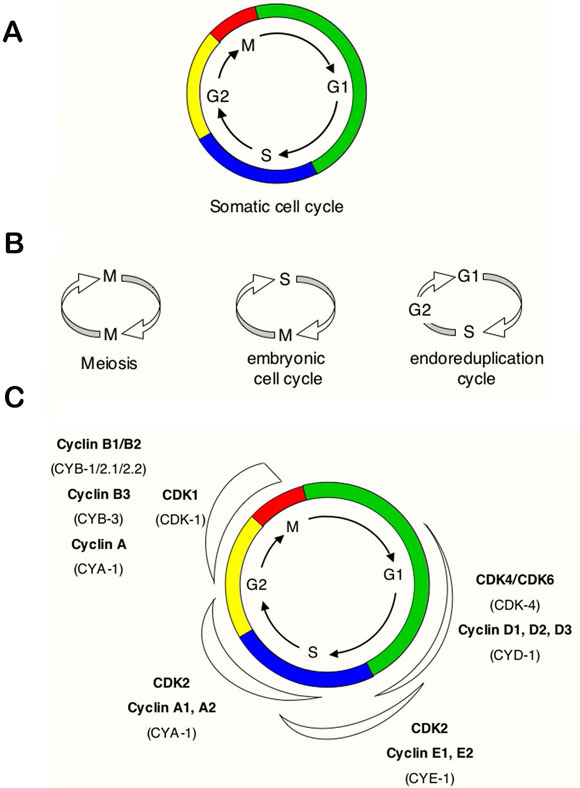
Animal development from a single-cell zygote to fertile adult requires many rounds of cell division. During each division, cells complete an ordered series of events that collectively form the "cell cycle". This cycle includes accurate duplication of the genome during the DNA synthesis phase (S phase), and segregation of complete sets of chromosomes to each of the daughter cells in M phase (Figure 1A). The somatic cell cycle also contains "Gap" phases, known as G1, which connects the completion of M phase to initiation of S phase in the next cycle, and G2, which separates the S and M phases. Dependent on environmental and developmental signals, cells in G1 may temporarily or permanently leave the cell cycle and enter a quiescent or arrested phase known as G0.
During development, variations of this typical somatic division cycle are used to fulfill specific requirements (Figure 1B). These include rapid embryonic cell cycles that lack G1 and G2 phases, meiotic cell cycles that allow formation of haploid gametes, and endoreduplication (or: "endoreplication") cycles in which S phases are not followed by mitosis. These variant cell cycles form part of the stereotypical pattern of cell divisions during C. elegans development.
Cell external signals and cell intrinsic information together determine whether cells enter a division cycle. In general, external signals affect this decision only until cells commit to go through the entire cycle, at a time in G1 known as "START" in yeast and "Restriction point" in mammals. From there on, progression through the cell cycle is controlled intrinsically by the cell-cycle machinery. The basic components of this machinery are conserved in all eukaryotes.
The collective results from studies in various eukaryotes have demonstrated that progression through the cell-division cycle is driven by activation and inactivation of cyclin-dependent kinases (CDKs), which trigger the transition to subsequent phases of the cycle. CDKs are small serine/threonine protein kinases that require association with a cyclin subunit for their activation. In yeast, a single CDK (p34CDC28 in Saccharomyces cerevisiae and p34cdc2 in Schizosaccharomyces pombe) acts with different cyclins to promote progression through G1, S and G2/M. Metazoans, including C. elegans, use not only a variety of cyclins but also multiple catalytic subunits (Figure 1C). Geminin inhibits DNA replication during S, G2, and M phases and that geminin destruction at the metaphase–anaphase transition permits replication in the succeeding cell cycle.
Product List
| Target | Catalog# | Product Name | Reactivity | Application |
|---|---|---|---|---|
CDK2 | CDK2 Rabbit Monoclonal antibody | Human,Mouse,Rat | WB,IHC,IF,IP,ELISA | |
Cdc2 (phospho Tyr15) | Cdc2 (phospho Tyr15) Rabbit Polyclonal Antibody | Human,Mouse,Rat,Monkey | WB,ELISA | |
Geminin | Geminin Rabbit Monoclonal Antibody | Human | WB |
Related Products
Super-sensitive ECL chemiluminescent reagent
References
- Cell-cycle regulation. van den Heuvel S. WormBook. 2005. [PMID: 18050422]
- Evolution of cyclin-dependent kinases (CDKs) and CDK-activating kinases (CAKs): differential conservation of CAKs in yeast and metazoa. Liu, J., et al. Mol. Biol. Evol. 2000. [PMID: 10889219]
- Cyclin D involvement demarcates a late transition in C. elegans embryogenesis. Yanowitz, J.,et al. Dev. Biol. 2005. [PMID: 15708572]
- Molecular mechanisms controlling the cell cycle: fundamental aspects and implications for oncology. Viallard JF, et al. Cancer Radiother. 2001. [PMID: 11355576]
- Regulation of Geminin Functions by Cell Cycle-Dependent Nuclear-Cytoplasmic Shuttling. Luo, L., et al. Molecular and Cellular Biology. 2007. [PMID: 17470552]

