Introduction to Escherichia coli (E. coli) Recombinant Protein Expression System
Escherichia coli (E. coli) is one of the most widely used prokaryotic expression systems for the production of recombinant proteins. Its popularity stems from several key advantages, including ease of genetic manipulation, rapid growth rate, and cost-effectiveness. E. coli has been extensively studied and optimized for protein expression, making it a cornerstone in both research and industrial applications.
Advantages of E. coli Expression System
High Efficiency and Yield
E. coli can grow rapidly with a doubling time of approximately 20 minutes under optimal conditions. This rapid growth allows for the production of large quantities of recombinant proteins in a relatively short period. The yield of recombinant proteins can often reach several grams per liter of culture, which is highly advantageous for industrial-scale production.
Cost-Effectiveness
The use of E. coli as a host organism is relatively inexpensive compared to other expression systems. The culture conditions are simple, requiring only basic nutrients and standard laboratory equipment. This makes E. coli an attractive choice for both academic research and commercial applications.
Genetic Manipulability
E. coli is highly amenable to genetic engineering. The well-characterized genetics and molecular biology of E. coli allow for easy insertion, deletion, and modification of genes. This flexibility enables researchers to optimize expression vectors, promoters, and regulatory elements to achieve high levels of protein production.
Scalability
E. coli-based protein expression systems can be easily scaled up from small laboratory cultures to large-scale industrial fermentation processes. This scalability ensures that the same expression system can be used for both initial research and commercial production, reducing the need for extensive re-optimization.
Expression Vector and Promoter Systems
Expression Vectors
E. coli expression systems typically utilize plasmid vectors that carry the gene of interest. These vectors often contain elements such as a bacterial origin of replication, a selectable marker (e.g., antibiotic resistance gene), and a strong promoter to drive the expression of the target gene. Commonly used vectors include pET, pGEX, and pMAL series, each designed for specific types of proteins and fusion tags.
Promoter Systems
Inducible promoters are widely used in E. coli expression systems to control the timing and level of protein expression. The most commonly used inducible promoter is the T7 promoter, which is activated by the addition of an inducer such as isopropyl-β-D-thiogalactopyranoside (IPTG). This allows for tight regulation of protein expression, preventing potential toxicity to the host cells during the growth phase and enabling high-level expression upon induction.
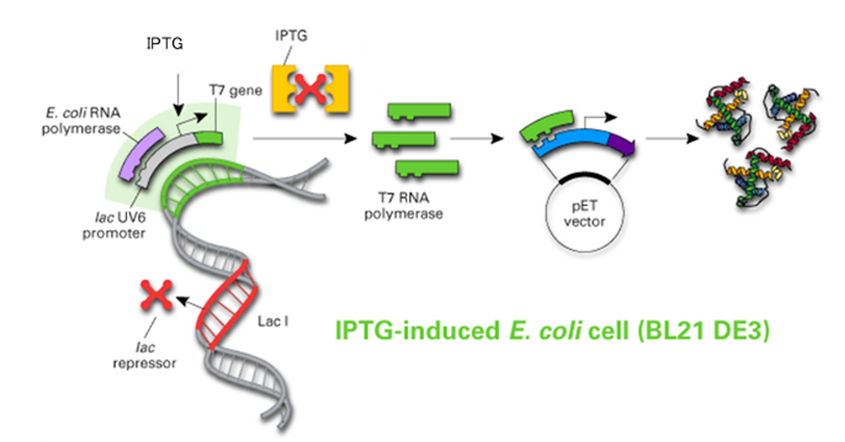
Applications
Research and Development
E. coli is extensively used for the production of recombinant proteins for structural and functional studies. It is suitable for generating proteins that do not require complex post-translational modifications (PTMs). Examples include enzymes, antibodies, and other proteins used in biochemical assays and structural biology.
Industrial Enzymes
Many industrial enzymes, such as proteases, lipases, and amylases, are produced using E. coli expression systems. These enzymes are used in various applications, including food processing, detergents, and biofuel production.
Therapeutic Proteins
E. coli has been used to produce several therapeutic proteins, such as insulin and growth hormones. However, for proteins that require extensive PTMs (e.g., glycosylation), alternative expression systems may be necessary.
Limitations
Post-Translational Modifications
E. coli lacks the machinery for complex PTMs, such as glycosylation and phosphorylation, which are often required for the proper folding and function of eukaryotic proteins. This limitation restricts the use of E. coli for producing certain mammalian proteins that rely on these modifications.
Protein Solubility and Inclusion Bodies
In some cases, recombinant proteins produced in E. coli may form inclusion bodies, which are insoluble aggregates of misfolded proteins. This can complicate protein purification and may require additional steps, such as solubilization and refolding, to obtain functional proteins.
Toxicity to Host Cells
Some recombinant proteins may be toxic to E. coli cells, leading to reduced cell viability and lower protein yields. This issue can be mitigated by optimizing expression conditions or using alternative bacterial hosts (such as C41, C43 strain) that are more tolerant of toxic proteins.
The E. coli recombinant protein expression system remains a powerful and versatile tool for producing a wide range of proteins. Its advantages in terms of efficiency, cost, and genetic flexibility make it a preferred choice for many applications. However, researchers must carefully consider the specific requirements of their target proteins, particularly regarding PTMs and solubility, to determine whether E. coli is the most suitable expression system. Despite its limitations, ongoing advancements in genetic engineering and protein folding technologies continue to enhance the capabilities of E. coli for recombinant protein production.
 | Dylan Z Dylan Z is a protein & antibody expert at EnkiLife, proficient in protein expression systems and antibody preparation techniques. He strives for excellence in technology and is committed to developing stable and user-friendly products for users. |
