How Indirect ELISA Works
The indirect ELISA (Enzyme-Linked Immunosorbent Assay) is a highly sensitive and versatile immunological technique primarily used to detect the presence of specific antibodies (e.g., antibodies produced after a pathogen infection, autoantibodies) in a sample. It can also be used for quantitative analysis. Its core principle relies on the use of an enzyme-labeled secondary antibody for signal amplification and detection, allowing for the indirect observation of an antigen-antibody reaction.
Basic Principle Overview
The basic process of an indirect ELISA is as follows: a known antigen is first immobilized onto a solid surface (typically a 96-well plate). The test sample containing the primary antibody (the target antibody to be detected) is then added. If present, the primary antibody will bind specifically to the antigen. Next, an enzyme-labeled secondary antibody is added. This secondary antibody is designed to recognize and bind to the primary antibody. Finally, a colorless substrate for the enzyme is added. The enzyme catalyzes a reaction that converts the substrate into a colored product. The intensity of this color, measured as optical density (OD), is used to determine the presence and concentration of the target antibody.
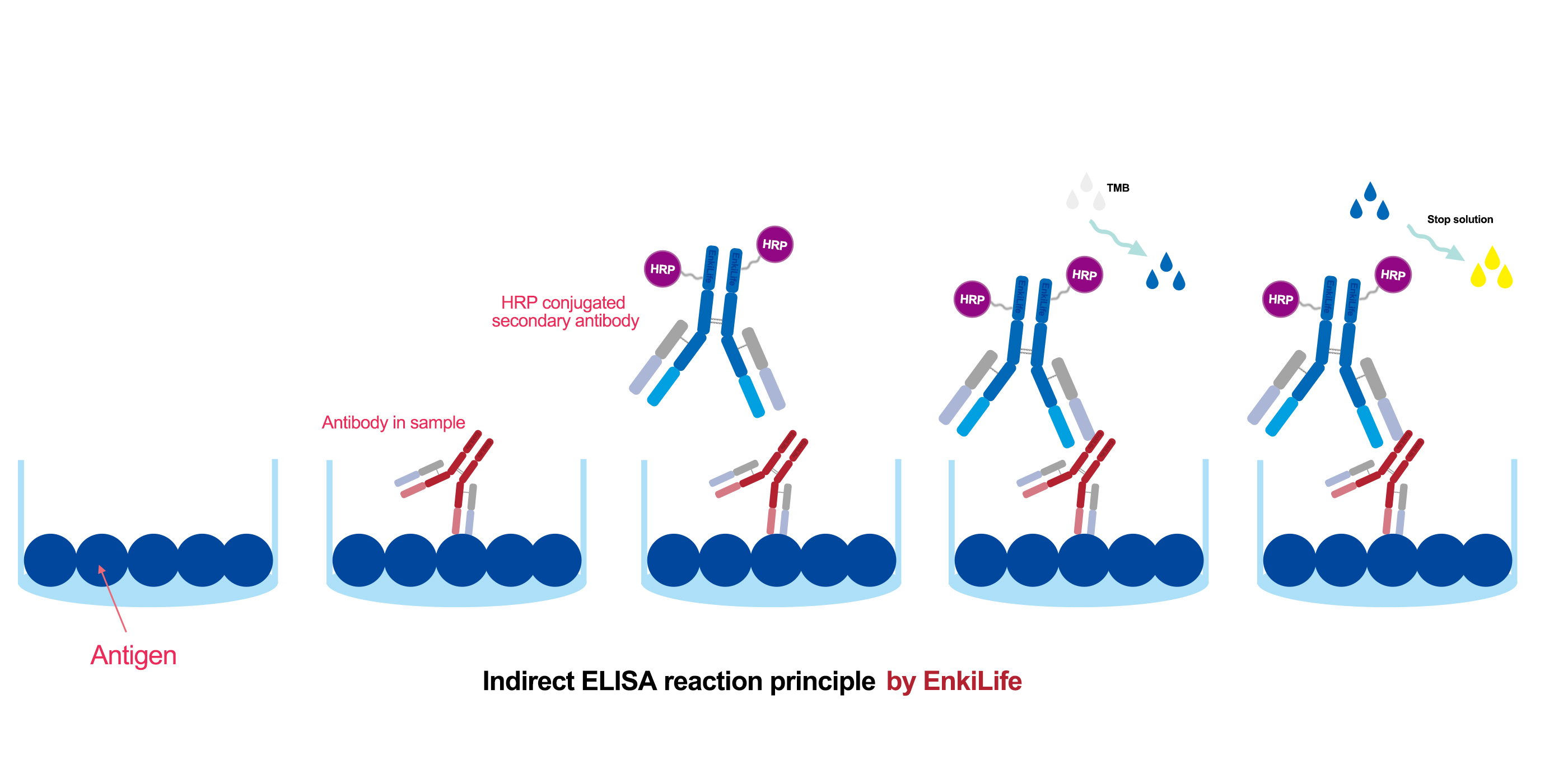
Step-by-Step Breakdown
Step 1: Coating
A purified, known antigen is dissolved in an alkaline coating buffer (e.g., carbonate-bicarbonate buffer) and added to the wells of a microplate. The plate is incubated, often at 4°C overnight or at 37°C for several hours. The antigen passively adheres to the plastic surface via hydrophobic interactions. The plate is then washed to remove any unbound antigen.
Step 2: Blocking
The surface of the well will still have uncoated binding sites, which could non-specifically bind proteins in subsequent steps, causing high background noise. A blocking agent (such as Bovine Serum Albumin (BSA), non-fat dry milk, or casein) is added to cover these vacant sites. After incubation, the plate is washed. This step is critical for reducing non-specific binding and ensuring a low background signal.
Step 3: Sample Addition and Incubation
The test sample (e.g., serum, plasma, cell culture supernatant—potentially containing the target primary antibody) is added, along with standards and controls. During incubation, if specific antibodies are present, they will bind to the immobilized antigen, forming an antigen-antibody complex. The plate is washed thoroughly to remove all unbound antibodies and other components.
Step 4: Addition of Enzyme-Labeled Secondary Antibody
An enzyme-labeled secondary antibody is added and the plate is incubated. This antibody is raised against the Fc region of the primary antibody (e.g., if the primary antibody is a mouse IgG, the secondary antibody would be an enzyme-labeled goat anti-mouse IgG). The secondary antibody binds specifically to the primary antibody that is already bound to the antigen, forming an "antigen-primary antibody-enzyme-labeled secondary antibody" sandwich. The plate is washed again to remove any unbound secondary antibody.
Step 5: Substrate Addition and Color Development
A colorless substrate solution is added. The choice of substrate depends on the enzyme used (e.g., TMB for Horseradish Peroxidase (HRP), pNPP for Alkaline Phosphatase (AP)). The enzyme catalyzes a reaction that converts the substrate into a colored product (e.g., TMB produces a blue solution, which turns yellow after stopping the reaction with an acid). The intensity of the color is proportional to the amount of primary antibody bound (i.e., the amount of target antibody in the sample).
Step 6: Detection and Analysis
A microplate reader is used to measure the Optical Density (OD or absorbance) of each well at a specific wavelength (e.g., 450 nm for stopped TMB).
Qualitative Analysis: The OD value of the test sample is compared to that of a negative control. A value significantly higher than the negative control indicates a positive result.
Quantitative Analysis: A standard curve is plotted using the OD values from samples of known concentration. The concentration of the target antibody in the test sample is then accurately calculated by interpolating its OD value from this curve.
Key Characteristics of Indirect ELISA
High Sensitivity: A single primary antibody can bind multiple enzyme-labeled secondary antibody molecules, resulting in significant signal amplification. This makes it more sensitive than direct ELISA (where the primary antibody itself is labeled).
High Versatility and Cost-Effectiveness: A single type of enzyme-labeled secondary antibody can be used to detect any primary antibody from the same species. For example, one HRP-labeled goat anti-human IgG antibody can be used in countless experiments to detect any human IgG primary antibody. This eliminates the need to label every primary antibody individually, saving time and resources.
Wide Application: It is the gold-standard method for antibody detection, widely used in disease diagnosis (e.g., HIV, HCV antibody tests), immunology research, and vaccine development.
Summary
In summary, indirect ELISA transforms an invisible antibody-binding event into a quantifiable colorimetric signal through a cascade of "antigen capture - primary antibody binding - signal amplification via enzyme-labeled secondary antibody - substrate conversion." Its key advantages of high sensitivity and exceptional versatility make it an indispensable tool in research and clinical diagnostics.
