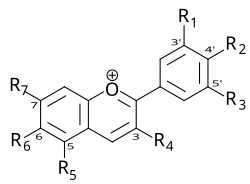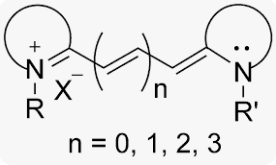
CY stands for Cyanine, which is a molecule with multiple methyl bridging chains between two nitrogen atoms and dispersed charges. Although Cyanine is etymologically derived from the blue hue, the Cyanine family encompasses a variety of dye types in the electromagnetic spectrum ranging from near-infrared to ultraviolet regions. Classic cyanine dyes contain two nitrogen-containing heterocycles, with conjugated chains composed of carbon hydrogen (CH) n inside the molecule, where n can be odd or even. Due to its structure, dyes have extremely high extinction coefficients, typically exceeding 100000 L/(mol * cm). Different substituents within the molecule can control the characteristics of anthocyanins, such as absorption wavelength, photostability, and fluorescence. For example, the absorption wavelength and fluorescence wavelength can be controlled by selecting the length of the polymerized vinylidene unit: longer cyanine dyes have higher absorption and emission wavelengths, reaching the near-infrared region (as shown in Figure 1). Based on the number of carbon atoms in the chain, cyanine dyes are classified into three groups: one methyl (CY1, n=0), three methyl (CY3, n=1), five methyl (CY5, n=2), and seven methyl (CY7, n=3). In addition to cell imaging, they are also commonly used in biological screening, protein immunoblotting, biomedical imaging, small animal in vivo imaging, etc.
| Name | Chromophore | Carbon Atoms Between Chromophores | Name | Chromophore | |
|---|---|---|---|---|---|
| n=0 | CY1 |  | |||
| n=1 | CY3 | 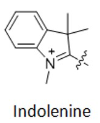 | 3 | CY3.5 | 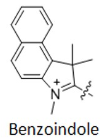 |
| n=2 | CY5 | 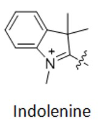 | 5 | CY5.5 | 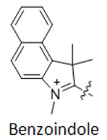 |
| n=3 | CY7 |  | 7 | CY7.5 |  |
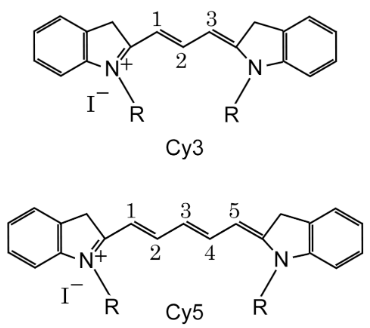
Figure 1 Examples of structural formulas of different cyanine dyes
Another classification method can be based on the number of carbon atoms between two indoline groups, where Cy represents "cyanine dye", CY3 represents three carbon atoms between two indoline groups, CY5 represents five carbon atoms between two indoline groups, and so on (as shown in Figure 2). However, CY2 dye is an exception (as shown in Figure 3), as it is an oxygen derivative rather than an indoline, but the oxygen derivative contains three carbon atoms, which needs to be distinguished from CY3.
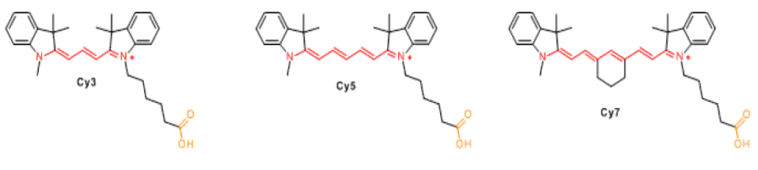
Figure 2 CY3, CY5, CY7 structural formulas
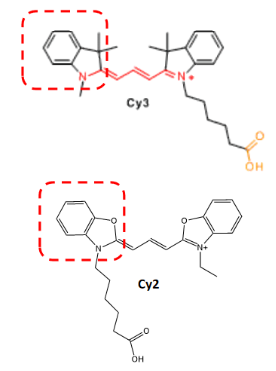
Figure 3 Comparison of CY2 and CY3 structural formulas
For dyes such as Cy3.5, Cy5.5, and Cy7.5, the difference from CY3, Cy5, and Cy7 is that the indoline group is replaced with a structure containing a benzo [a] group (as shown in Figure 4). Structural changes can alter the fluorescence properties of molecules, allowing several fluorescent groups to cover most of the visible and near-infrared spectral ranges (as shown in Figure 5).
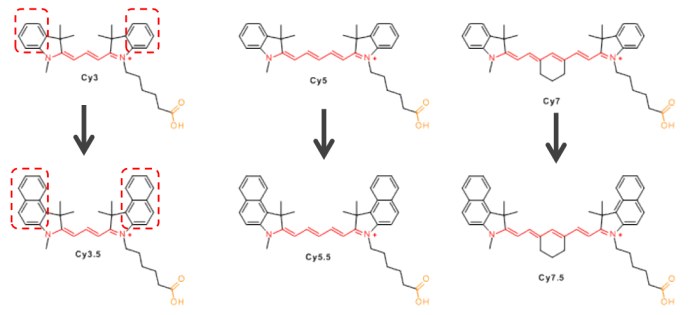
Figure 4 Comparison of CY3/CY3.5, CY5/CY5.5, CY7/CY7.5 structural formulas
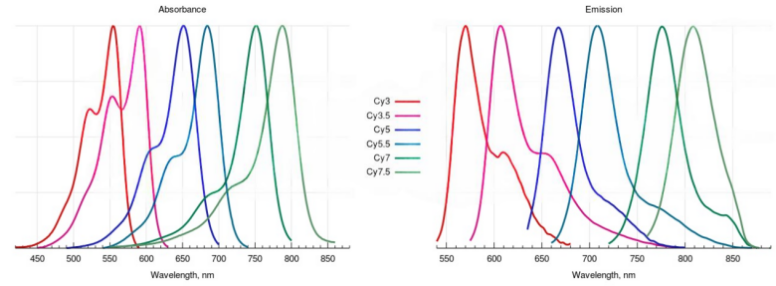
Figure 5 Spectral analysis of commonly used cyanine dyes
Cyanine dyes were first synthesized over a century ago. Initially, they were used to expand the sensitivity range of photographic emulsions—specifically, to broaden the wavelength range capable of forming images—thereby enhancing the color richness of film-based images. Consequently, they were also employed in CD-R and DVD-R media. The types primarily used were green or pale blue dyes, but these exhibited chemical instability. Recent cyanine dyes incorporate stabilizers, typically metal atoms bound to the cyanine dye molecules, which significantly slow down degradation and improve dye stability. The archival lifespan of optical discs using these dyes is generally 75 years or longer. In the early 1990s, Alan Waggoner and his colleagues at Carnegie Mellon University (CMU) introduced the magenta dyes that later became highly popular in the life sciences. These dyes were modifications of Indocyanine Green (ICG), a magenta dye used in angiography since the 1970s, and all contain two indolenine rings flanking a polymethylene chain. These dyes were found to have low non-specific binding to biomolecules and exhibit bright fluorescence due to their high molar extinction coefficients and favorable quantum yields. The designations such as Cy3 and Cy5 were first proposed by Ernst et al. in 1989. These designations are non-standard, as they provide no indication of the dyes’ chemical structures. In the original paper, the numerical suffixes represented the number of methylene groups (as shown in Figure 1), while the side chains were unspecified. This ambiguity has led to references in the literature to various structures being labeled as Cy3 or Cy5. The R groups (in their structure) do not need to be identical; in the dyes actually used, these are short aliphatic chains with highly reactive groups—such as N-hydroxysuccinimide or methylene carboxylic acid—attached at one or both ends. Although the patent protection for standard Cy series dyes has expired, the trademark for the "Cy" designation remains in place. As a result, dyes that are structurally identical to Cy dyes but sold under different names now exist, as do dyes marketed under Cy designations but with different chemical structures (as shown in Figure 7). A variety of analogues of the standard Cy2/3/3.5/5/5.5/7/7.5 dyes have been developed through numerous modification methods, including Alexa Fluor dyes, DyLight dyes, FluoProbes dyes, Sulfo-Cy dyes, Seta dyes, and IRIS dyes. These dyes are interchangeable with Cy dyes in most biochemical applications and offer improvements in properties such as solubility, fluorescence, or photostability (as shown in Figure 6). The structural formulas of some of these optimized dyes have been published following patent expiration, while those of others remain undisclosed due to ongoing patent protection.
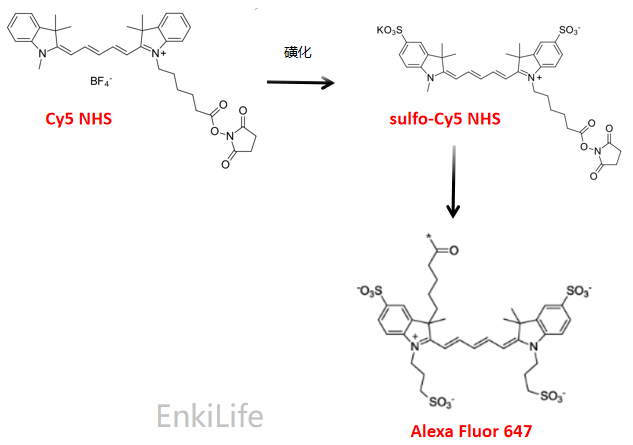
Figure 6 Evolution and development diagram of cyanine dyes (with the same main chain structure and optimized and improved side chains)
| Dye Name | Maximum Excitation/Emission(nm) | Extinction Coefficient ε, L/(mol*cm) | Quantum Yield |
|---|---|---|---|
| Cy5 | 646/662 | 250000 | 0.2 |
| Sulfo-Cy5 | 646/662 | 271000 | 0.28 |
| Alexa Fluor 647 | 655/680 | 191800 | 0.15 |
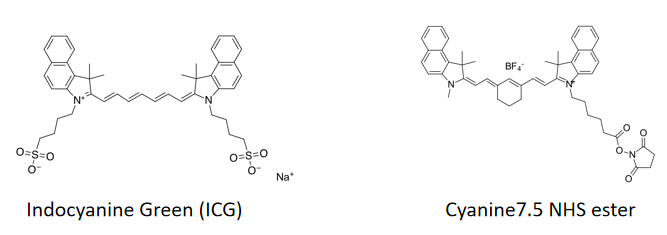
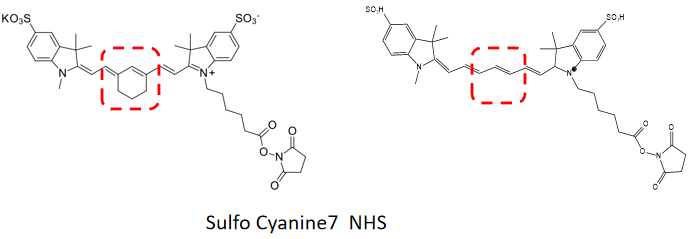
Figure 7 Comparison of the structural evolution and development of several similar dyes (for example, dyes with the same name as Sulfo Cyanine7 NHS, but with certain differences in molecular chemical structure)
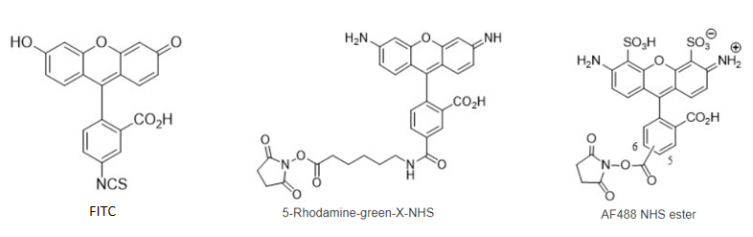
Figure 8 Comparison of the structural formulas of three approximate dyes FITC, Rhodamine, and Alexa Fluor488
| Dye Name | Maximum Excitation/Emission(nm) | Extinction Coefficient ε, L/(mol*cm) | Quantum Yield |
|---|---|---|---|
| Cy2 | 490/510 | 150000 | 0.12 |
| Cy3 | 550/566 | 162000 | 0.1 |
| Alexa Fluor 488 | 495/519 | 71800 | 0.91 |
| FITC | 492/520 | 74000 | 0.92 |
| Rhodamine 110 | 496/522 | 82000 | 0.79 |
CY dyes are categorized into non-sulfonated cyanine dyes and sulfonated cyanine dyes, and both exhibit very similar fluorescence properties (as shown in Figure 9). However, there are some notable differences in their labeling protocols: (1)Non-sulfonated cyanine dyes must be dissolved in organic solvents (dimethylformamide, DMF; or dimethyl sulfoxide, DMSO) before use and then added to the aqueous buffer solution of the target molecule. The recommended cosolvent volumes are as follows: 10% for Cy3, Cy5, and Cy7, and 15% for the counterparts of Cy3.5, Cy5.5, and Cy7.5. Sulfonated dyes contain additional sulfonic acid groups, which facilitate the dissolution of dye molecules in the aqueous phase. The negatively charged sulfonic acid groups reduce the aggregation of conjugates when the dye is highly labeled, and the negative charge can minimize non-specific binding to non-target positively charged biomolecules (such as certain membrane surface proteins). (2)Differences in purification: When dialysis purification is performed using aqueous buffer solutions, sulfonated cyanine dyes must be used to achieve effective removal of unreacted dye materials. The reactions involving both sulfonated and non-sulfonated cyanine dyes can be purified by gel filtration, chromatography (high-performance liquid chromatography, HPLC; fast protein liquid chromatography, FPLC; or ion exchange), or electrophoresis. (3)In the labeling of DNA and proteins, the two types of dyes are interchangeable, and both can be used for labeling biomolecules. Sulfonated cyanine dyes, which are water-soluble and less prone to aggregation in water, are the preferred choice for antibody and protein labeling. Non-sulfonated cyanine dyes have lower dependence of their fluorescence properties on solvents and the surrounding environment.
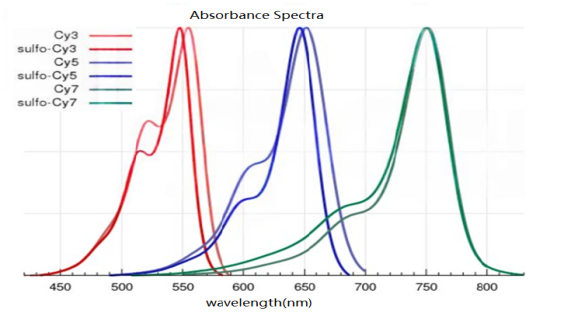
Figure 9 Spectroscopy of Sulfonated and Non Sulfonated Cyanine Dyes
EnkiLife provides a variety of sulfonated cyanine dyes and small molecule fluorescent dye labeling kits similar to cyanine dyes. The labeling of antibodies is simple, reliable, fast, and easy to use. The labeled protein antibodies can be used for various subsequent applications, such as immunofluorescence, flow cytometry, ELISA, etc.
| Dye Name | Kit Item Number | Ex max/Em max | Product Specifications | Relative Fluorescence Brightness | Fluorescent Color |
|---|---|---|---|---|---|
| Cyanine2 | RE80019 | 490nm/510nm | 40ug/200ug/2mg | ★ | Green |
| Cyanine3 | RE80007p | 548nm/563nm | 40ug/200ug/2mg | ★ | Yellow |
| Cyanine5 | RE80008p | 646nm/662nm | 40ug/200ug/2mg | ★★★ | Red |
| Cyanine5.5 | RE80020 | 675nm/694nm | 40ug/200ug/2mg | ★★ | Crimson |
| Cyanine7 | RE80021 | 750nm/773nm | 40ug/200ug/2mg | ★★ | Crimson |
| FITC | RE80001p | 495nm/520nm | 40ug/200ug/2mg | ★★ | Green |
| Fluorescein-X | RE80022 | 495nm/519nm | 40ug/200ug/2mg | ★★ | Green |
| Fluor350 | RE80009p | 346nm/445nm | 40ug/200ug/2mg | ★ | Blue |
| Fluor405 | RE80010p | 400nm/424nm | 40ug/200ug/2mg | ★ | Purple |
| Fluor430 | RE80023 | 430nm/545nm | 40ug/200ug/2mg | ★ | Yellow-Green |
| Fluor488 | RE80011p | 490nm/513nm | 40ug/200ug/2mg | ★★ | Green |
| Fluor532 | RE80024 | 530nm/555nm | 40ug/200ug/2mg | ★★ | Yellow-Green |
| Fluor555 | RE80012p | 555nm/572nm | 40ug/200ug/2mg | ★ | Yellow |
| Fluor568 | RE80025 | 578nm/602nm | 40ug/200ug/2mg | ★★ | Orange |
| Fluor590 | RE80018 | 590nm/620nm | 0.5mg/1.25mg/2.5mg | ★ | Orange |
| Fluor594 | RE80013p | 590nm/617nm | 40ug/200ug/2mg | ★★ | Orange |
| Fluor647 | RE80014p | 651nm/668nm | 40ug/200ug/2mg | ★★★ | Red |
| Fluor680 | RE80015p | 680nm/702nm | 40ug/200ug/2mg | ★★ | Crimson |
| Fluor700 | RE80026 | 702nm/723nm | 40ug/200ug/2mg | ★★ | Crimson |
| Fluor750 | RE80017p | 747nm/770nm | 40ug/200ug/2mg | ★ | Crimson |
| Fluor770 | RE80016 | 770nm/790nm | 0.5mg/1.25mg/2.5mg | ★ | Crimson |
In daily life, we often hear about another type of anthocyanin dye, and its Chinese name is often mixed with the name of Cyanine, which is used for fluorescent labeling and development. In order to distinguish between the two, further explanation will be given.
1. Basic Definition and Source
| Category | Anthocyanins | Cyanine |
|---|---|---|
| Nature | Natural plant pigments (flavonoid compounds) | Synthetic dyes (cyanine dye family, containing nitrogen heterocyclic structures) |
| Sources | Plant cell sap from purple cabbage, blueberries, grape skins, etc. | Laboratory chemical synthesis (such as indocyanine green, Cy series dyes) |
| Chemical Structure | Based on cyanidin core, containing polyphenolic hydroxyl groups and glycosyl groups
| Containing polymethine chains (-CH=CH- chains) and aromatic heterocycles (such as indole)
|
2. Color development mechanism and stability
(1) Color development principle:
Anthocyanins: Dependent on pH changes, they alter their conjugated structure through intramolecular proton transfer (such as acid turning red, alkaline turning blue).
Cyanidin: Due to the π - electron delocalization of the poly (methyl) alkyne chain, the absorption wavelength is long (near-infrared region), and the color is affected by substituents and solvents.
(2) Stability:
Anthocyanins are easily destroyed by light, heat, and metal ions (need to be stored at low temperatures and away from light).
Cyanidin has high stability, but some derivatives (such as CY5) are prone to photobleaching and require the addition of anti quenching agents.
3. Comparison of application fields
| Category | Anthocyanins | Cyanine |
|---|---|---|
| Food Industry | Natural coloring agents (juice, candy), pH indicators | Not applicable (synthetic dyes are prohibited for use in food) |
| Biomedicine | Antioxidants (health supplements), anti-inflammatory research | Fluorescent labeling (DNA sequencing, in vivo imaging), photodynamic therapy |
| Textile/Chemical Industry | Traditional plant dyeing (requires fixing with a mordant) | Synthetic fiber dyeing (such as CY dye for acrylic fiber), optical disc dyeing |
4. Safety and Environmental Impact
anthocyanin: High safety, edible and non-toxic; Highly biodegradable and environmentally friendly.
Cyanidin: Experimental grade dyes may be toxic to aquatic organisms (subject to certain regulations).
5. Common Misconceptions and Relevance
Name confusion: Because the Chinese transliteration is similar ("green" and "cyanide"), but the two are not chemically related.
Cross functional: Anthocyanins are occasionally used in simple pH test strips (natural substitutes); Cyanidin derivatives (such as Indocyanine Green) can be used for medical imaging and are not related to the biological activity of anthocyanins.
6. summary
Anthocyanins and anthocyanins are fundamentally different: the former is a natural antioxidant pigment evolved from plants, while the latter is a functional dye synthesized by humans. Understanding their differences can help avoid application confusion (such as the prohibition of adding synthetic cyanine dyes in food), while accurately selecting materials in biomedical and industrial fields.
Related Products
Super-sensitive ECL chemiluminescent reagent
References
[1] H.A.Shindy.Fundamentals in the chemistry of cyanine dyes: A review. Dyes and Pigments. Volume 145, October 2017, Pages 505-513.
[2] G.S. Gopika, P.M. Hari Prasad. Chemistry of cyanine dyes-A review.Materials Today: Proceedings,2020, 2214-7853.
[3] Aria Vahdani,Mehdi Moemeni, Daniel Holmes. Mechanistic Insight into the Thermal “Blueing” of Cyanine Dyes.J.Am. Chem. Soc. 2024, 146, 19756- 19767.
[4] Elliot. Inhibition of glutathione reductase by flavonoids. A structure-activity study.Biochem Pharmacol 1992;44(8): 1603-8.
[5] Igarashi K, et al. Preventive effects of dietary cabbage acylated anthocyanins on paraquat-induced oxidative stress in rats. Biosci Biotechnol Biochem 2000 Aug;64(8):1600-7.