Actin Nucleation and Polymerization
Actin Nucleation and Polymerization
Actin is one of the most abundant proteins in eukaryotic cells and is a key component of the cytoskeleton, the internal framework of the cell. Its most remarkable characteristic is its dynamic nature—it can rapidly assemble and disassemble, thereby driving cell movement and changes in cell shape.
Core Principle: Actin Polymerization Kinetics
Actin filaments have structural polarity, meaning that their two ends are chemically and kinetically different:
Barbed End (+ End): The end that grows more rapidly, usually pointing towards the cell membrane or the direction of movement.
Pointed End (- End): The end that grows more slowly, usually pointing towards the interior of the cell.
The polymerization process follows a classic "treadmilling" model, but more importantly, it involves three key kinetic parameters:
Nucleation: This is the rate-limiting step. Assembling the initial few G-actin monomers into a stable oligomer "seed" is very difficult and slow. Once nucleation occurs, subsequent polymerization becomes much faster.
Elongation: G-actin monomers are added to or released from both ends of the filament.
Treadmilling: Under a certain monomer concentration, the rate of net addition of monomers at the barbed end equals the rate of net loss of monomers at the pointed end. The total length of the filament remains constant, but monomers move like on a "conveyor belt," being added at the barbed end and released at the pointed end.

Stage 1: Nucleation - G-actin monomers collide to form unstable trimer nucleus
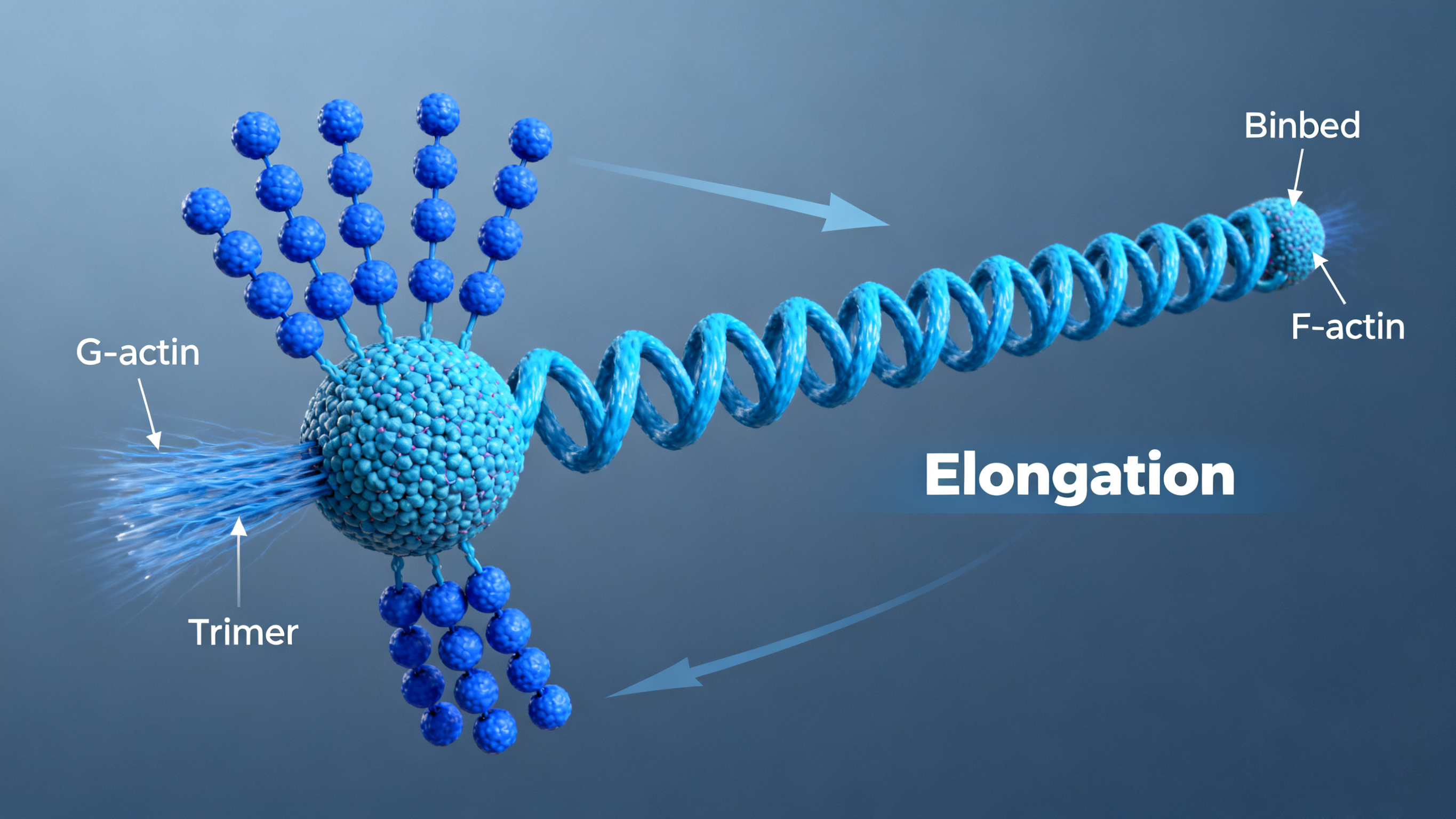
Stage 2: Elongation - Monomers add to both ends with faster growth at barbed end
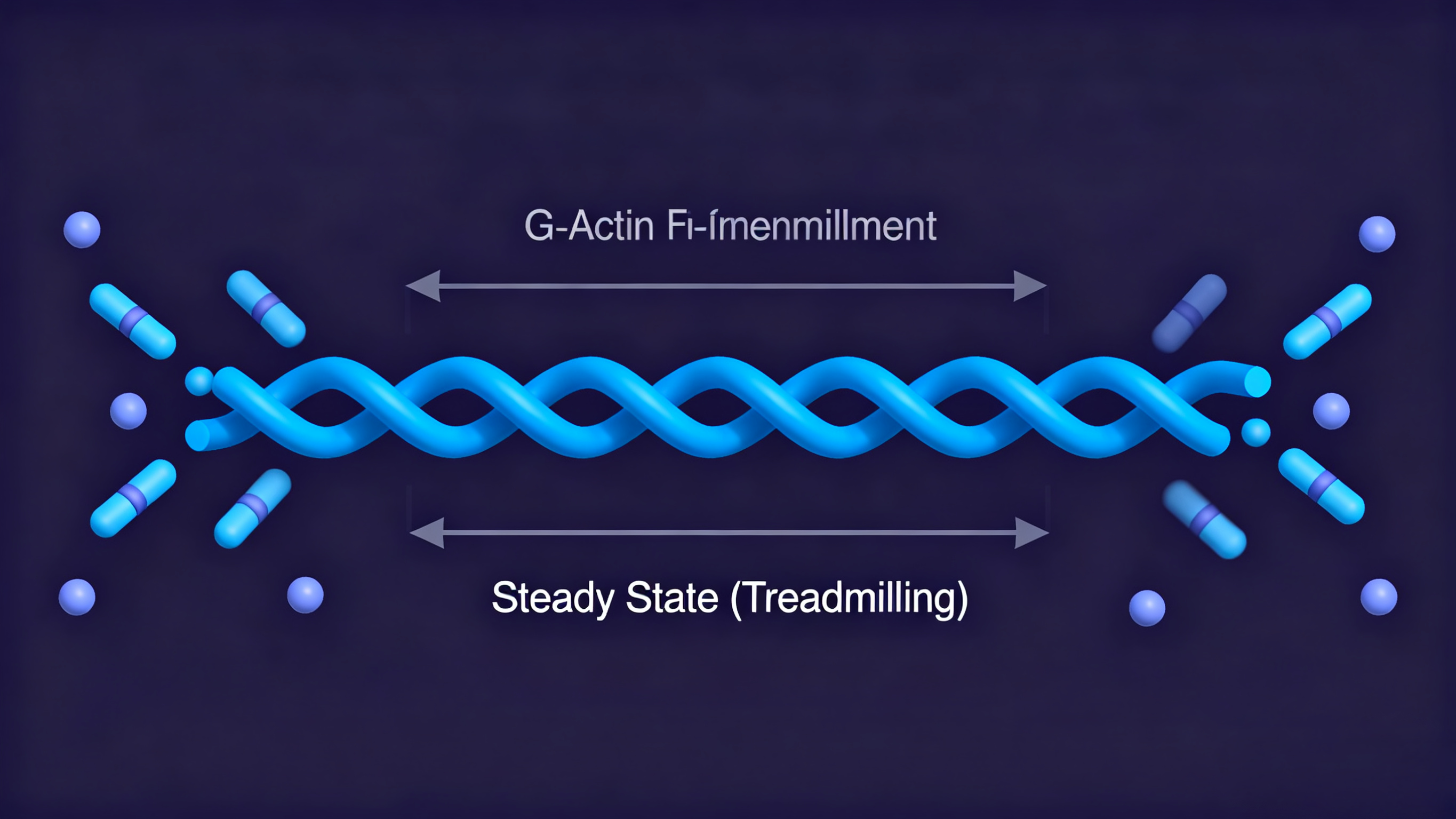
Stage 3: Steady state - Polymerization and depolymerization reach dynamic equilibrium
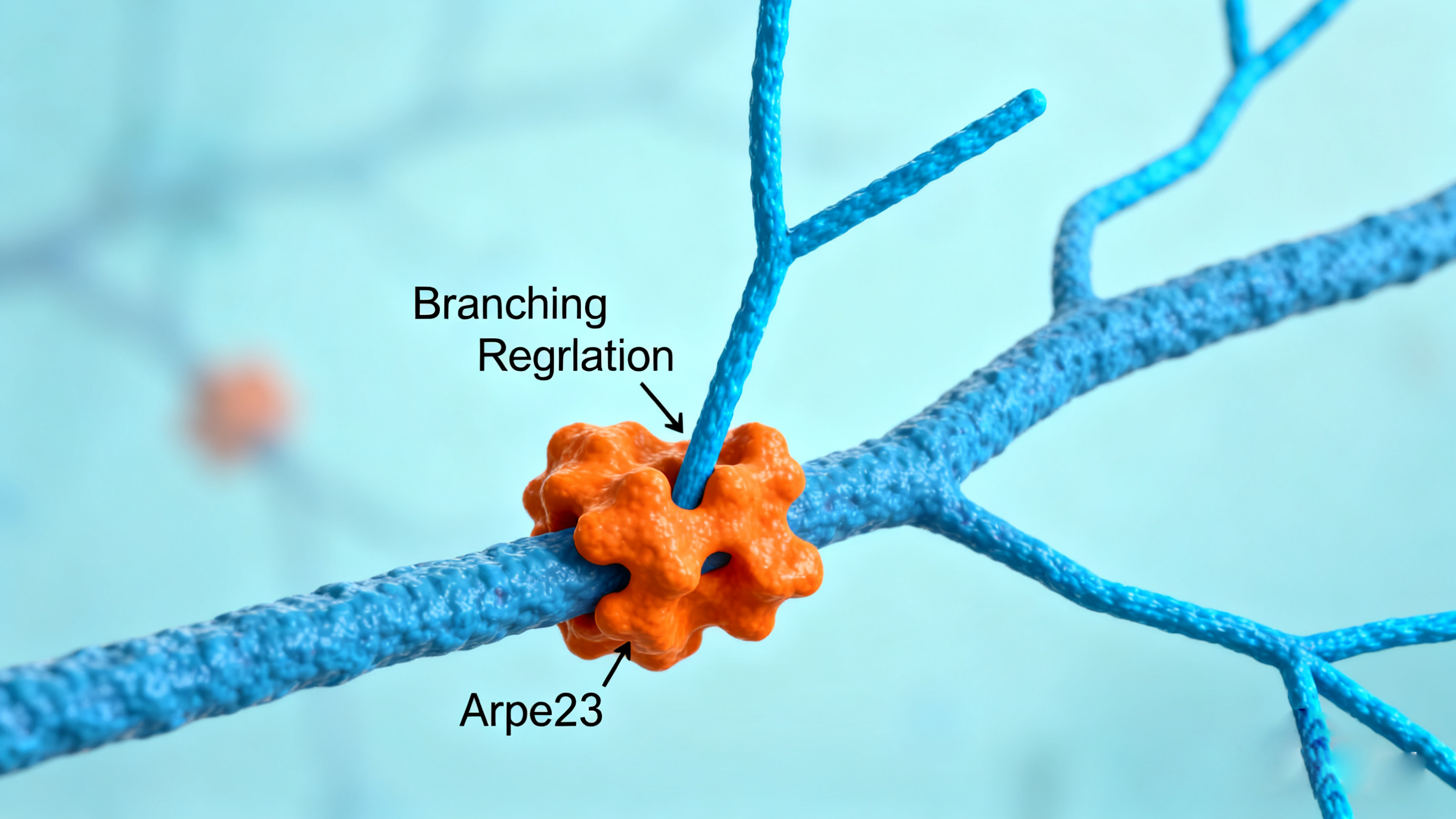
Stage 4: Regulation - Nucleation-promoting factors induce branching
Key Regulation: Actin-Binding Proteins
Cells do not passively allow actin to polymerize at will. They precisely control actin polymerization in space and time through more than 150 different actin-binding proteins. These proteins are mainly divided into the following categories:
| Functional Category | Representative Proteins | Mechanism of Action |
|---|---|---|
| Nucleation Factors | ARP2/3 Complex | Initiates polymerization, especially adept at nucleating on the side of existing filaments to form branched networks (e.g., lamellipodia). |
| Formins Proteins | Initiates polymerization, continuously binds to the barbed end, promoting the formation of linear, unbranched filaments (e.g., stress fibers, contractile rings). | |
| Capping Proteins | CapZ (barbed end), Tropomodulin (pointed end) | Binds to the ends of filaments, preventing monomer addition or loss, stabilizing filament length. |
| Depolymerization/ Severing Factors | Cofilin/ADF | Cuts old actin filaments and promotes their depolymerization, increasing the number of barbed ends, providing sites for new polymerization. |
| Monomer-Binding Proteins | Profilin | Binds to G-actin, promotes its addition to the barbed end, but inhibits spontaneous nucleation. |
| Thymosin β4 | Sequesters G-actin, forming a polymerization reservoir, preventing premature polymerization. | |
| Crosslinking Proteins | α-Actinin, Filamin | Bundles or crosslinks actin filaments into different structures, such as bundles or three-dimensional gel-like networks, providing mechanical strength. |
| Motor Proteins | Myosin | Uses ATP as energy to "walk" along actin filaments, generating mechanical force, the source of power for muscle contraction and cell migration. |
Major Biological Functions of Actin
Based on the above dynamic and regulatory principles, the actin cytoskeleton undertakes crucial functions:
- Maintaining Cell Shape and Mechanical Strength: Acts as an internal scaffold for the cell, resisting external pressure.
- Cell Migration: This is the most classic function. The cell front promotes actin polymerization through ARP2/3 complex, forming lamellipodia or filopodia, propelling the cell forward.
- Cytokinesis: At the end of cell division, actin and myosin form a contractile ring, which tightens like a rope, splitting the cell into two.
- Intracellular Transport: The actin network works with myosin to transport organelles, vesicles, and other cargo in the cell cortex.
- Cell Cortex Formation: The dense actin network beneath the cell membrane provides support for the membrane and participates in vesicle transport (e.g., endocytosis).
- Cell Adhesion: Connects with adhesion molecules such as cadherins, participating in the formation of intercellular adhesions.
Recommendations for Actin Antibodies
| Target | Catalog# | Product Name | Application | Reactivity |
|---|---|---|---|---|
| Arp2 | AMRe84444 | Arp2 Rabbit Monoclonal Antibody | WB,IHC,ICC,IF | Human,Mouse,Rat |
| Arp3 | APRab07160 | Arp3 Rabbit Polyclonal Antibody | WB,ELISA | Human,Mouse,Rat |
| Cofilin | AMRe04121 | Cofilin Rabbit Monoclonal Antibody | WB,IHC-F,IHC-P,ICC/IF | Human |
| p-Cofilin | APRab04483 | Cofilin (phospho Ser3) Rabbit Polyclonal Antibody | WB,IHC-P,IF-P,IF-F,ICC/IF,ELISA | Human,Mouse,Rat |
| Profilin 1 | AMRe01506 | Profilin 1 Rabbit Monoclonal Antibody | WB,IHC-F,IHC-P,ICC/IF,IP | Human,Mouse,Rat |
| β-actin | AMRe80020 | β-actin Rabbit Monoclonal Antibody | WB,ELISA | Human,Mouse,Rat |
Related Products
References
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–263.
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212.
- Riedl J, Crevenna AH, Kessenbrock K, et al. Lifeact: a versatile marker to visualize F-actin. Nat Methods. 2008;5:605–607.
- Rottner K, Faix J, Bogdan S, Linder S, Kerkhoff E. Actin assembly mechanisms at a glance. J Cell Sci. 2017;130:3427–3435.
 | Voisey Voisey is a technical support specialist at EnkiLife, proficient in immunology and cell biology. She is committed to providing customers with professional and efficient technical support. Additionally, she is involved in research on customers' fields of study and designs highly cost-effective solutions for them. |
