Size2:50μg price2:$378
Size3:500μg price3:$1890
| Name | Recombinant Human HO-1 |
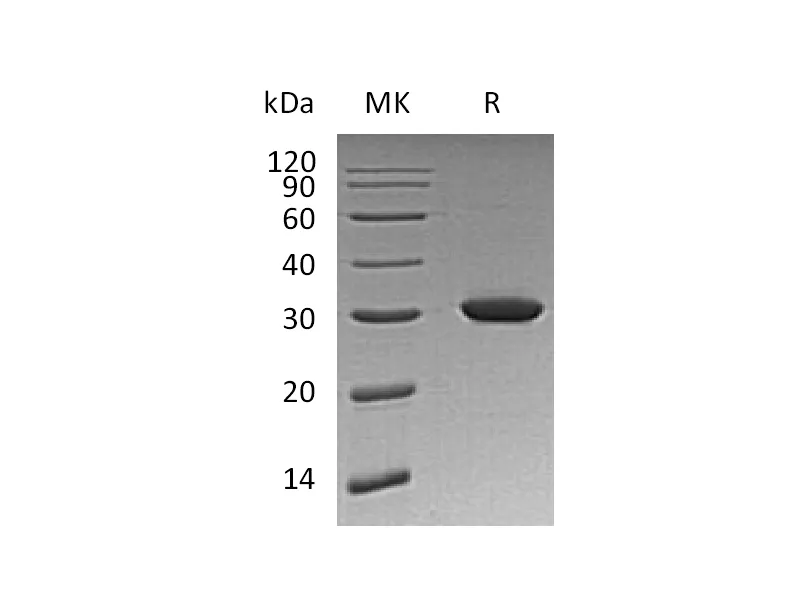
| Purity | Greater than 95% as determined by reducing SDS-PAGE |
| Endotoxin level | <1 EU/µg as determined by LAL test. |
| Construction | Recombinant Human Heme Oxygenase 1 is produced by our E.coli expression system and the target gene encoding Met1-Thr261 is expressed. |
| Accession # | P09601 |
| Host | E.coli |
| Species | Human |
| Predicted Molecular Mass | 29.86 KDa |
| Buffer | Supplied as a 0.2 μm filtered solution of 20mM PB, 150mM NaCl, 1mM EDTA, pH 7.4. |
| Form | Liquid |
| Shipping | The product is shipped on dry ice/polar packs.Upon receipt, store it immediately at the temperature listed below. |
| Stability&Storage | Store at ≤-70°C, stable for 6 months after receipt.Store at ≤-70°C, stable for 3 months under sterile conditions after opening. Please minimize freeze-thaw cycles. |
Alternative Names
Heme Oxygenase 1; HO-1; HMOX1; HO; HO1
Background
Heme Oxygenase 1 (HO-1) is an enzyme in endoplasmic reticulum that belongs to the heme oxygenase family. HO-1 cleaves the heme ring at the alpha methene bridge to form Biliverdin. Biliverdin is subsequently converted to Bilirubin by Biliverdin reductase. In physiological state, the highest activity of HO-1 is found in the spleen, where senescent erythrocytes are sequestrated and destroyed. HO-1 activity is highly inducible by its substrate heme and by various non-heme substances such as heavy metals, bromobenzene, endotoxin, oxidizing agents and UVA. HO-1 is involved in the regulation of cardiovascular function and response to a variety of stressors. Defects in HO-1 are the cause of Heme Oxygenase 1 deficiency, resulting in marked erythrocyte fragmentation and intravascular hemolysis, coagulation abnormalities, endothelial damage, and iron deposition in renal and hepatic tissues.
Note
For Research Use Only , Not for Diagnostic Use.
