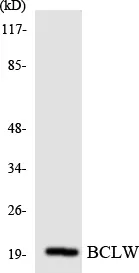
Size:50μL Price:$118
Size:100μL Price:$220
Size:200μL Price:$380
Application:WB,IHC,ICC/IF,ELISA
Reactivity:Human,Mouse,Rat
Conjugate:Unconjugated
Optional conjugates: Biotin, FITC (free of charge). See other 26 conjugates.
Gene Name:LAMA3
Summary
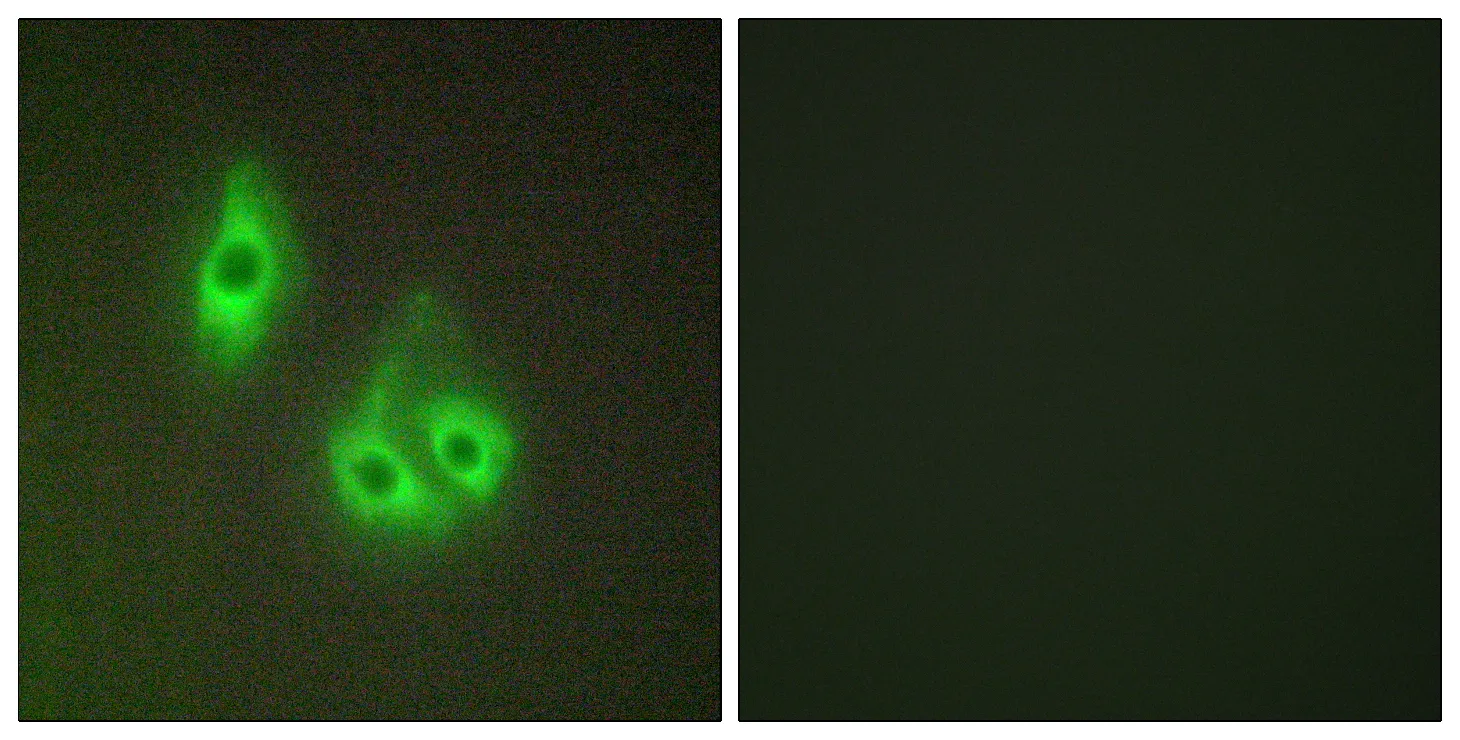
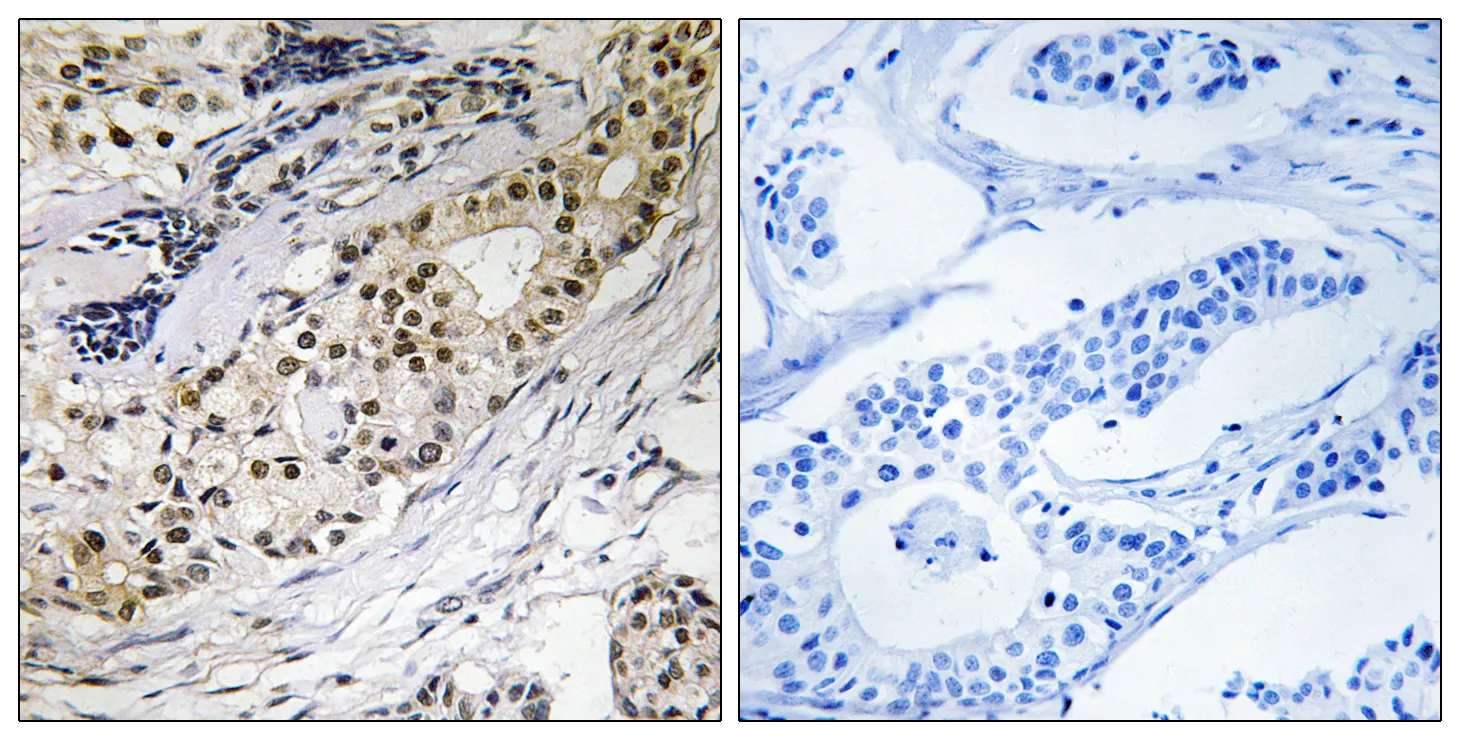
Performance
Immunogen
Application
Background
The protein encoded by this gene belongs to the laminin family of secreted molecules. Laminins are heterotrimeric molecules that consist of alpha, beta, and gamma subunits that assemble through a coiled-coil domain. Laminins are essential for formation and function of the basement membrane and have additional functions in regulating cell migration and mechanical signal transduction. This gene encodes an alpha subunit and is responsive to several epithelial-mesenchymal regulators including keratinocyte growth factor, epidermal growth factor and insulin-like growth factor. Mutations in this gene have been identified as the cause of Herlitz type junctional epidermolysis bullosa and laryngoonychocutaneous syndrome. Alternative splicing and alternative promoter usage result in multiple transcript variants. [provided by RefSeq, Dec 2014],disease:Defects in LAMA3 are a cause of epidermolysis bullosa junctional Herlitz type (H-JEB) [MIM:226700]; also known as junctional epidermolysis bullosa Herlitz-Pearson type. JEB defines a group of blistering skin diseases characterized by tissue separation which occurs within the dermo-epidermal basement membrane. H-JEB is a severe, infantile and lethal form. Death occurs usually within the first six months of life. Occasionally, children survive to teens. H-JEB is marked by bullous lesions at birth and extensive denudation of skin and mucous membranes that may be hemorrhagic.,disease:Defects in LAMA3 are the cause of laryngoonychocutaneous syndrome (LOCS) [MIM:245660]. LOCS is an autosomal recessive epithelial disorder confined to the Punjabi Muslim population. The condition is characterized by cutaneous erosions, nail dystrophy and exuberant vascular granulation tissue in certain epithelia, especially conjunctiva and larynx.,domain:Domain G is globular.,domain:The alpha-helical domains I and II are thought to interact with other laminin chains to form a coiled coil structure.,function:Binding to cells via a high affinity receptor, laminin is thought to mediate the attachment, migration and organization of cells into tissues during embryonic development by interacting with other extracellular matrix components.,function:Laminin-5 is thought to be involved in (1) cell adhesion via integrin alpha-3/beta-1 in focal adhesion and integrin alpha-6/beta-4 in hemidesmosomes, (2) signal transduction via tyrosine phosphorylation of pp125-FAK and p80, (3) differentiation of keratinocytes.,induction:Laminin-5 is up-regulated in wound sites of human skin.,similarity:Contains 1 laminin IV type A domain.,similarity:Contains 1 laminin N-terminal domain.,similarity:Contains 15 laminin EGF-like domains.,similarity:Contains 5 laminin G-like domains.,subcellular location:Major component.,subunit:Laminin is a complex glycoprotein, consisting of three different polypeptide chains (alpha, beta, gamma), which are bound to each other by disulfide bonds into a cross-shaped molecule comprising one long and three short arms with globules at each end. Alpha-3 is a subunit of laminin-5 (epiligrin/kalinin/nicein), and possibly also a component of laminin-6 (K-laminin) and laminin-7 (KS-laminin).,tissue specificity:Skin; respiratory, urinary, and digestive epithelia and in other specialized tissues with prominent secretory or protective functions. Epithelial basement membrane, and epithelial cell tongue that migrates into a wound bed. A differential and focal expression of the subunit alpha-3 is observed in the CNS.,
Research Area
Focal adhesion;ECM-receptor interaction;Pathways in cancer;Small cell lung cancer;
