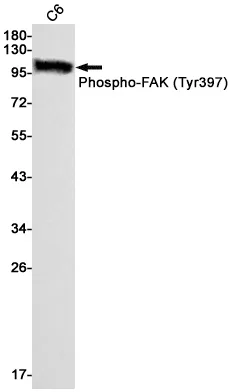
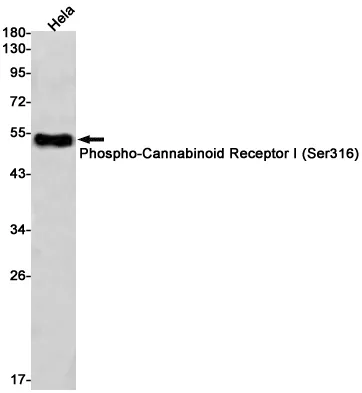
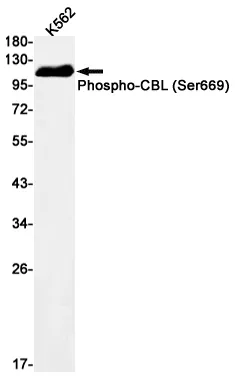
Summary
Performance
Immunogen
Application
Background
Converts arachidonate to prostaglandin H2 (PGH2), a committed step in prostanoid synthesis. Constitutively expressed in some tissues in physiological conditions, such as the endothelium, kidney and brain, and in pathological conditions, such as in cancer. PTGS2 is responsible for production of inflammatory prostaglandins. Up-regulation of PTGS2 is also associated with increased cell adhesion, phenotypic changes, resistance to apoptosis and tumor angiogenesis. In cancer cells, PTGS2 is a key step in the production of prostaglandin E2 (PGE2), which plays important roles in modulating motility, proliferation and resistance to apoptosis. Dual cyclooxygenase and peroxidase in the biosynthesis pathway of prostanoids, a class of C20 oxylipins mainly derived from arachidonate, with a particular role in the inflammatory response.
Research Area
Cardiovascular