An Introduction to Antibodies
An Introduction to Antibodies
Antibodies, also known as immunoglobulins (Ig), are a class of highly specific glycoproteins produced by the immune system, particularly by B lymphocytes, in response to antigenic stimulation. They are the core effector molecules of adaptive humoral immunity and play an irreplaceable role in defending against pathogen infections, clearing abnormal cells, and maintaining homeostasis within the body.
I. Basic Structure and Functional Domains
The basic structure of an antibody molecule is characterized by a classic "Y" - shaped four - peptide - chain symmetrical structure:
-Two identical heavy chains (Heavy Chain, H chain): With a larger molecular weight (approximately 50-75 kDa), the heavy chains determine the class and subclass of the antibody.
-Two identical light chains (Light Chain, L chain): With a smaller molecular weight (approximately 25 kDa), light chains are divided into kappa (κ) and lambda (λ) types.
-Interchain disulfide bonds: These bonds connect the heavy and light chains (H-L) as well as the two heavy chains (H-H), maintaining the stability of the molecular conformation.
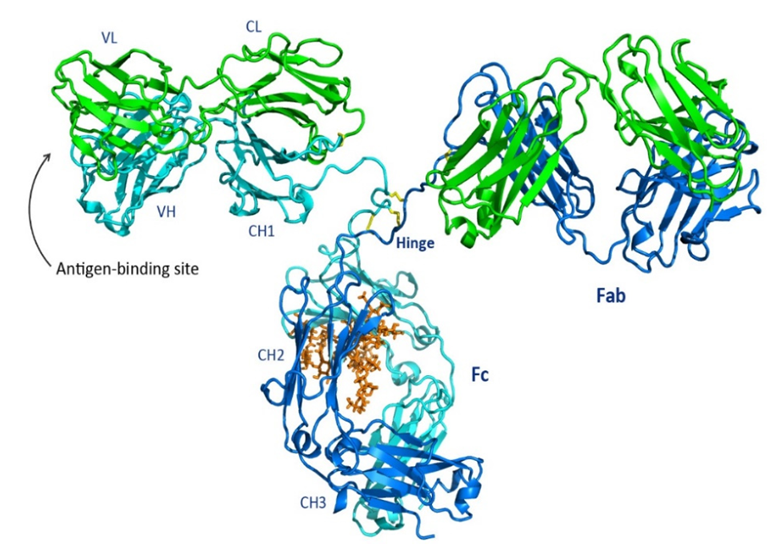
Antibody molecules can be hydrolyzed into characteristic fragments by proteases (such as papain and pepsin):
-Fab fragment (Fragment antigen - binding): Comprising one complete light chain and the N-terminal portion of the heavy chain (VH and CH1 domains). Each Fab fragment has one antigen - binding site, which determines the specificity of the antibody. This site is formed by the variable region (Variable Region, V region) of both the light and heavy chains, with highly variable amino acid sequences that create unique complementarity-determining regions (CDRs) that directly contact and bind to antigenic epitopes.
-Fc fragment (Fragment crystallizable): Comprising the C- terminal portion of the two heavy chains (CH2 and CH3 domains for IgG, IgA, and IgD). The Fc fragment does not bind antigens but mediates the effector functions of antibodies, such as:
-Binding and activating the complement system (via the classical pathway).
-Binding to cells expressing Fc receptors (such as macrophages, NK cells, neutrophils, and mast cells), mediating opsonophagocytosis, antibody - dependent cell - mediated cytotoxicity (ADCC), and type I hypersensitivity reactions.
-Crossing the placenta (IgG) or being transported to mucosal surfaces (IgA).
II. Classes and Subclasses of Immunoglobulins
Based on the antigenicity and structure of the constant region (Constant Region, C region) of the heavy chains, mammalian antibodies are divided into five major classes (isotypes):
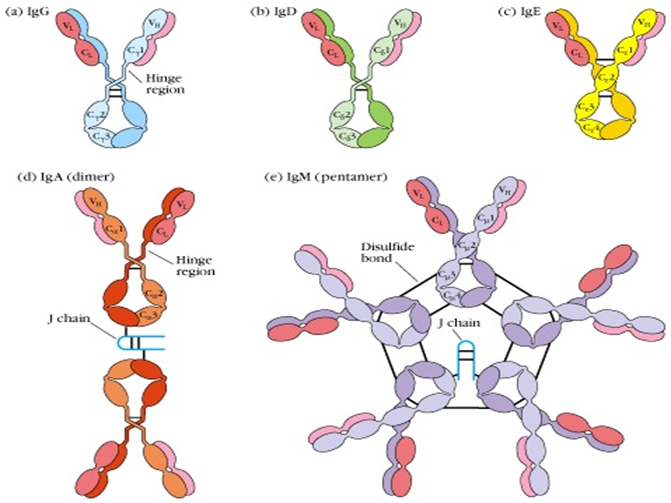
1. IgG: The most abundant in serum (70- 75%), with a molecular weight of approximately 150 kDa. It is the primary antibody in secondary immune responses, possessing strong toxin - and virus - neutralizing capabilities, and can activate complement, mediate opsonization, and ADCC. It is the only antibody that can cross the placenta, providing passive immunity to newborns. Human IgG is further divided into four subclasses based on subtle differences in the heavy chains: IgG1, IgG2, IgG3, and IgG4.
2. IgM: Exists primarily in a pentameric form (approximately 900 kDa), and is the first antibody produced during a primary immune response (the "pioneer antibody"). It has a strong ability to activate the classical complement pathway (due to its multiple Fc segments), playing a prominent role in early defense, clearance of pathogens, and immune complexes. Monomeric IgM is mainly expressed on the surface of B cell membranes, serving as the B cell receptor (BCR).
3. IgA: Predominantly monomeric in serum, but in mucosa - associated lymphoid tissue (MALT), it often forms dimers or trimers and combines with the secretory component to form secretory IgA (sIgA). sIgA is the main barrier of mucosal immunity, found in saliva, tears, respiratory and gastrointestinal secretions, and colostrum, preventing pathogens from colonizing and invading mucosal surfaces.
4. IgD: Exists in a monomeric form (approximately 180 kDa) with extremely low levels in serum. Its primary function is to serve as part of the antigen receptor (BCR) on the surface of mature B cells, participating in B cell activation, differentiation, and maintenance of immune tolerance.
5. IgE: Exists in a monomeric form (approximately 190 kDa) with the lowest levels in serum. Its Fc segment binds to the high - affinity FcεRI receptor on the surface of mast cells and basophils. Upon binding to the corresponding antigen (allergen), it triggers degranulation of these cells, releasing histamine and other bioactive mediators, mediating type I hypersensitivity reactions (allergic reactions) and anti - parasitic immunity.
III. Mechanisms of Antibody Diversity Generation
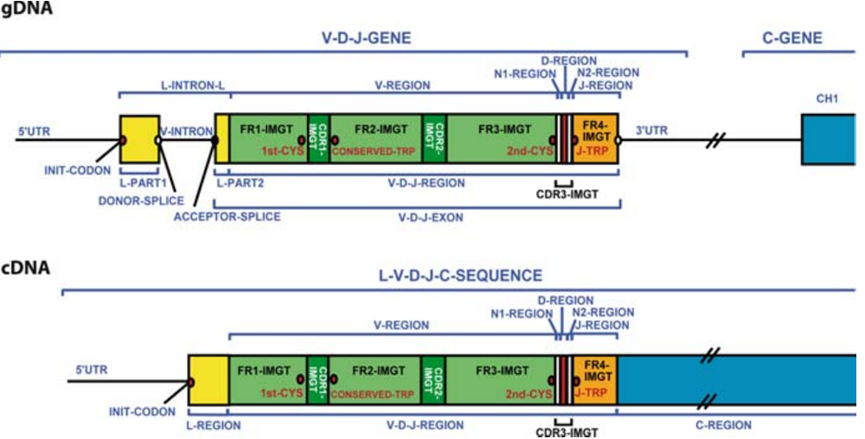
The antibody repertoire possesses near - infinite diversity, enabling the recognition of virtually all antigens in nature. This diversity arises from:
1. Combinatorial diversity from germline gene segment rearrangement: V(D)J gene rearrangement (heavy chain: VH, DH, JH segments; light chain: VL, JL segments) generates a vast array of different V - region gene combinations.
2. Junctional diversity: At the V - D, D - J, and V - J junctions, imprecise nucleotide excision and addition (P - nucleotides, N - nucleotides) cause sequence variations.
3. Somatic hypermutation: In germinal centers, activated B cells undergo high - frequency point mutations in their V - region genes, leading to affinity maturation through antigen - driven selection of high - affinity antibodies.
4. Random pairing of heavy and light chains: The random combination of different heavy and light chains further amplifies diversity.
5. Class switch recombination (CSR): Induced by cytokines and other signals, B cells can change the isotype of the antibodies they secrete (from IgM/IgD to IgG, IgA, or IgE) without altering antigen specificity (V region remains unchanged), thereby acquiring different effector functions to meet diverse immune needs.
IV.Core Functions of Antibodies
1. Specific recognition and binding of antigens: This is the most fundamental function of antibodies. Through their V - region CDRs, antibodies precisely recognize and bind to specific epitopes on antigens (such as pathogen surface proteins, toxins, exogenous macromolecules).
2. Neutralization: Antibodies bind to key sites on pathogens (such as viruses, bacterial toxins), preventing them from interacting with host cell receptors or entering cells, thereby neutralizing their infectivity or toxicity.
3. Opsonization: The Fab segment of antibodies (mainly IgG) binds to particulate antigens (e.g., pathogens), and its Fc segment binds to FcγR on phagocytic cells (macrophages, neutrophils), significantly enhancing the phagocytic efficiency of these cells.
4. Complement activation: Antibodies (mainly IgM and IgG1/IgG3) bound to antigens form immune complexes that effectively activate the classical pathway of the complement system, producing various effects: forming the membrane attack complex (MAC) to lyse pathogens or cells (lysis), generating opsonins (C3b) to enhance phagocytosis, and producing chemotactic factors (C3a, C5a) to recruit inflammatory cells.
5. Antibody - dependent cell - mediated cytotoxicity (ADCC): The Fab segment of antibodies (mainly IgG) binds to antigens on target cells (such as virus - infected cells or tumor cells), and its Fc segment binds to FcγRIII (CD16) on effector cells (e.g., NK cells), activating these effector cells to release perforin, granzymes, and other cytotoxic molecules to kill target cells.
6. Mediation of hypersensitivity reactions: IgE binds to FcεRI on mast cells/basophils, and upon allergen cross - linking, triggers degranulation and type I hypersensitivity reactions. Certain IgG/IgM can also participate in type II (cytotoxic) and type III (immune complex - mediated) hypersensitivity reactions.
7. Immunoregulation: Antibodies can regulate immune responses through the idiotype network. The Fc segment binding to FcγRIIB on B cells can transmit inhibitory signals, down - regulating B cell activity. Antibody feedback inhibition is also an important mechanism for regulating humoral immunity.
Antibodies are the immune system’s exquisitely precise and powerful arsenal. Their ingenious Y-shaped structure, especially the highly variable antigen-binding fragment (Fab) and the constant effector region (Fc), endows them with the ability to recognize an almost infinite variety of antigens and to perform diverse immune functions. As research tools, they play a crucial role in protein purification, cell or tissue localization studies (WB, IHC,IF,et.) and functional studies.
EnkiLife provides an extensive selection of high-quality antibodies to meet your diverse research needs. Search our catalogue of antibodies.
With the maturation of monoclonal antibody technology and the rapid development of antibody engineering, antibodies have evolved from objects of basic immunological research to a cornerstone of modern medical diagnostics, prevention, and therapy. They have demonstrated revolutionary therapeutic effects in major disease areas such as cancer and autoimmune diseases, and continue to lead the forefront of biomedical innovation.
 | Dylan Z Dylan Z is a protein & antibody expert at EnkiLife, proficient in protein expression systems and antibody preparation techniques. He strives for excellence in technology and is committed to developing stable and user-friendly products for users. |
