Introduction to Antibody Labeling
What is Antibody Labeling?
Antibody labeling, or antibody conjugation, involves attaching antibodies to specific tags to facilitate detection and measurement in various immunoassays.
Why Label Antibodies?
Antibodies lack intrinsic, observable physical signals, necessitating the use of tags for visualization. This process transforms specific antibody-antigen binding events into detectable signals, enabling the study of protein structure, function, localization, and interactions.
Visualization Mechanisms
Fluorescent Optical Signals: Fluorescein (e.g., FITC) emits fluorescence upon excitation.
Chemiluminescent Signals: Horseradish peroxidase (HRP) catalyzes luminescent substrates like luminol.
Absorbance Color Signals: HRP catalyzes chromogenic substrates like TMB.
Mass Spectrometry Signals: Metal tags (e.g., indium) are used in CyTOF.
Types of Antibody Labeling
1.Primary and Secondary Antibodies
Depending on the application and detection method, antibodies are classified as primary or secondary, corresponding to direct and indirect detection methods.
Classification | Definition | Biological Essence |
Primary Antibody | Binds specifically to the target antigen (e.g., protein, polysaccharide, pathogen) | Produced by host animals (e.g., mice, rabbits) via immunization-induced B cell clones |
Secondary Antibody | Targets the constant region (Fc segment) of the primary antibody, amplifying or converting the signal | Produced by immunizing against the primary antibody's host species IgG |
When labeling antibodies, tags bind to specified amino acids or chemical structures. If primary and secondary antibodies have similar molecular weights and structures, the labeling process differs little between them.
Example: Detecting human CD4 protein:
Primary antibody: Anti-human CD4 monoclonal antibody (mouse-derived IgG), binds to human CD4 antigen.
Secondary antibody: Goat anti-mouse IgG, binds to the Fc segment of the mouse-derived primary antibody.
2.Antibody Subclasses and Isotypes
Antibodies in Placentate mammals are classified based on structure and composition (heavy chain constant regions) into IgA, IgD, IgE, IgG, and IgM. Other species have different antibody types (e.g., IgZ in zebrafish, IgY in chickens). Understanding antibody types is crucial for accurate experimental results and troubleshooting. Labeling different antibody types may require optimization.
Example: Detecting mouse CD45R protein:
Option 1:
Primary antibody: Anti-mouse CD45R protein (rat-derived IgM), binds to mouse CD45R antigen.
Secondary antibody: Goat anti-rat IgM, binds to the Fc segment of the rat-derived primary antibody.
Option 2:
Primary antibody: Anti-mouse CD45R protein (rabbit-derived IgG), binds to mouse CD45R antigen.
Secondary antibody: Goat anti-rabbit IgG, binds to the Fc segment of the rabbit-derived primary antibody.
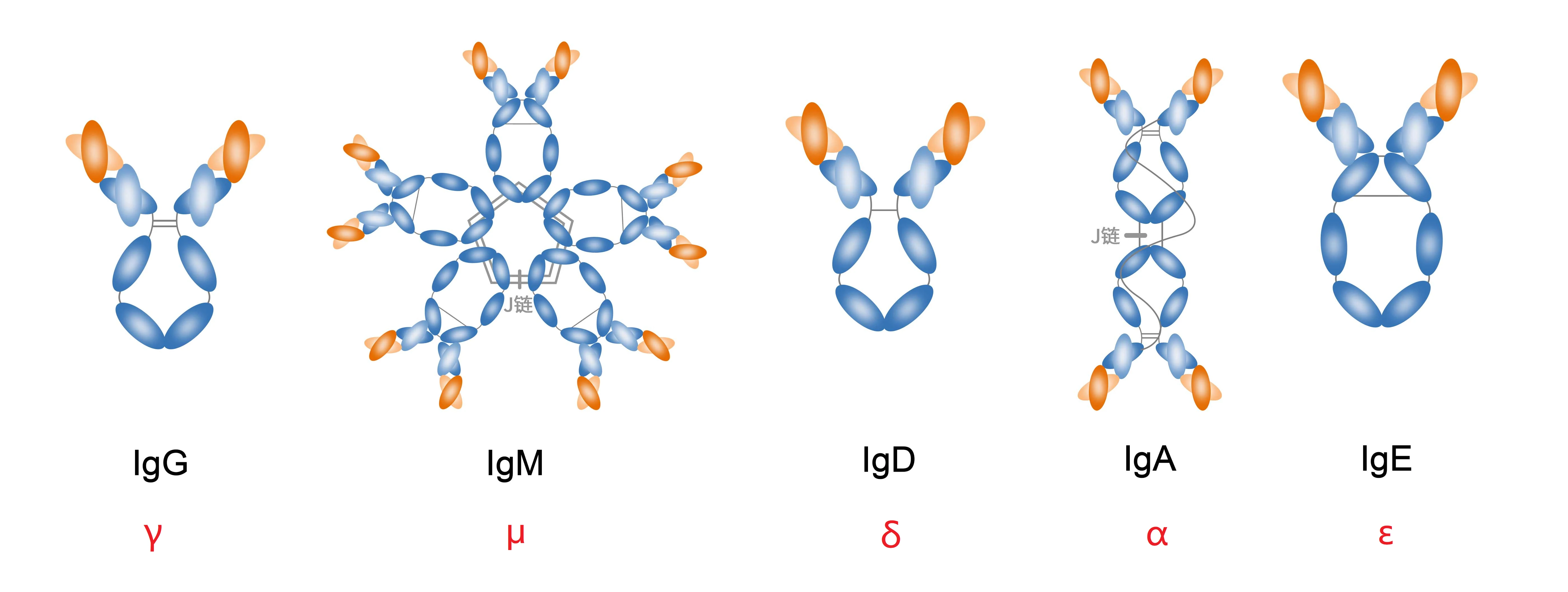
Human and Mouse IgG Subclasses
Human IgG can be further divided into IgG1, IgG2, IgG3, and IgG4, while mouse IgG is categorized into IgG1, IgG2a, IgG2b, and IgG3. These subclasses allow for the production of highly specific secondary antibodies, enhancing experimental accuracy.
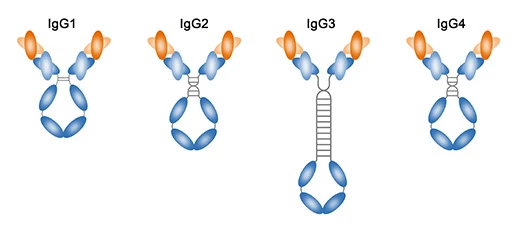
3.Antibody Clonality
Antibodies can be classified as monoclonal or polyclonal based on their B cell origin. Monoclonal antibodies, produced by identical B cell clones, bind to a single epitope and offer more consistent performance. Polyclonal antibodies, from heterogeneous B cell populations, bind to multiple epitopes on the same protein.
Example: Monoclonal antibodies specifically recognize a single epitope, while polyclonal antibodies recognize multiple epitopes on the same protein. Since polyclonal and monoclonal antibodies of the same type differ mainly in variable region amino acids, their labeling methods are nearly identical.
4.Recombinant Antibodies
Recombinant antibodies, produced via genetic engineering, offer known gene and peptide sequences, enabling large-scale production with high batch-to-batch consistency. When labeling recombinant antibodies, their amino acid sequences, structural information, and molecular weights must be considered, as some may have modified structures or fused tags requiring specific conjugation chemistry.
Immunological Applications of Labeled Antibodies
Common immunoassay techniques and associated tags:
Immunodetection Technique | Tags |
Flow Cytometry (FC) | Phycoerythrin(PE), Allophycocyanin (APC), tandem dyes, Fluor 488, etc. |
Immunocytochemistry/Immunofluorescence (ICC/IF) | Fluorescent dyes, HRP |
Immunohistochemistry (IHC) | Enzymes (HRP or AP), biotin |
Western Blotting (WB) | Enzymes (HRP or AP), fluorescent dyes |
Enzyme-Linked Immunosorbent Assay (ELISA) | Enzymes (HRP or AP), biotin |
The tags used in different immunodetection techniques are not strictly exclusive. For example, Fluor 488 fluorescent dye can be used to label antibodies for immunofluorescence, Western blotting, or fluorescent ELISA.
Methods of Antibody Labeling
Traditional covalent conjugation techniques, such as NHS ester methods or indirect labeling with bifunctional reagents, often involve coupling tags to lysine residues. These methods require substantial chemical knowledge and separation techniques to remove excess tags or low molecular weight activators. A deep understanding of antibody production and raw material control is also crucial for successful labeling.
In contrast, using EnkiLife's labeling kits simplifies the process. Users can follow the kit's instructions to obtain labeled antibodies without mastering complex conjugation chemistry. These kits help avoid common pitfalls in chemical conjugation, saving time and effort in experimental testing.
 | Hansun Hansun is a protein labeling expert at EnkiLife, proficient in immunology, labeling techniques, and flow cytometry, dedicated to precise protein labeling and empowering technological breakthroughs. Every protein deserves to be 'seen'. |
