Mammalian Protein Expression Systems: A Comprehensive Overview
Introduction
Mammalian protein expression systems are now widely used in the biopharmaceutical industry for producing complex recombinant proteins. These systems use mammalian cell lines like Chinese hamster ovary (CHO) cells and human embryonic kidney (HEK) 293 cells. They can generate proteins with intricate post-translational modifications (PTMs) and human-like molecular structures, making them highly suitable for clinical applications, including the production of therapeutic proteins and monoclonal antibodies.
Key Features
Accurate Post-Translational Modifications (PTMs)
Mammalian cells are capable of performing a wide range of PTMs, including glycosylation, phosphorylation, acetylation, and disulfide bond formation. These modifications are essential for the proper folding, stability, and functionality of proteins. The PTMs in mammalian systems closely resemble those found in humans, ensuring that the recombinant proteins exhibit native-like characteristics.
Native-Like Protein Folding
The cellular machinery in mammalian cells ensures that recombinant proteins are folded correctly. This is particularly important for proteins that require specific three-dimensional structures for their biological activity, such as monoclonal antibodies and hormone-like proteins.
High-Fidelity Functional Activity
Proteins produced in mammalian systems often display functional activities comparable to their natural counterparts. This is crucial for therapeutic applications where protein functionality is critical for efficacy.
Applications
Monoclonal Antibodies (mAbs)
Mammalian expression systems are critical for producing monoclonal antibodies (mAbs) used in immunotherapy and diagnostics. Examples include drugs like trastuzumab (Herceptin) and adalimumab (Humira). These antibodies are essential for targeted cancer therapies and autoimmune disease treatments.
Manufacturing Recombinant Protein Pharmaceuticals
Mammalian cell expression systems are used to produce various recombinant protein drugs, such as erythropoietin (EPO) and human growth hormone. These proteins require precise post-translational modifications to maintain their biological activity, making mammalian systems the preferred choice for their production.
Viral Antigens and Vaccines
Mammalian systems are also widely used to produce viral proteins for vaccines. For instance, they are crucial in the development of vaccines against human papillomavirus (HPV) and other viral diseases. The ability of mammalian cells to produce proteins with accurate PTMs and proper folding ensures the efficacy and safety of these vaccines.
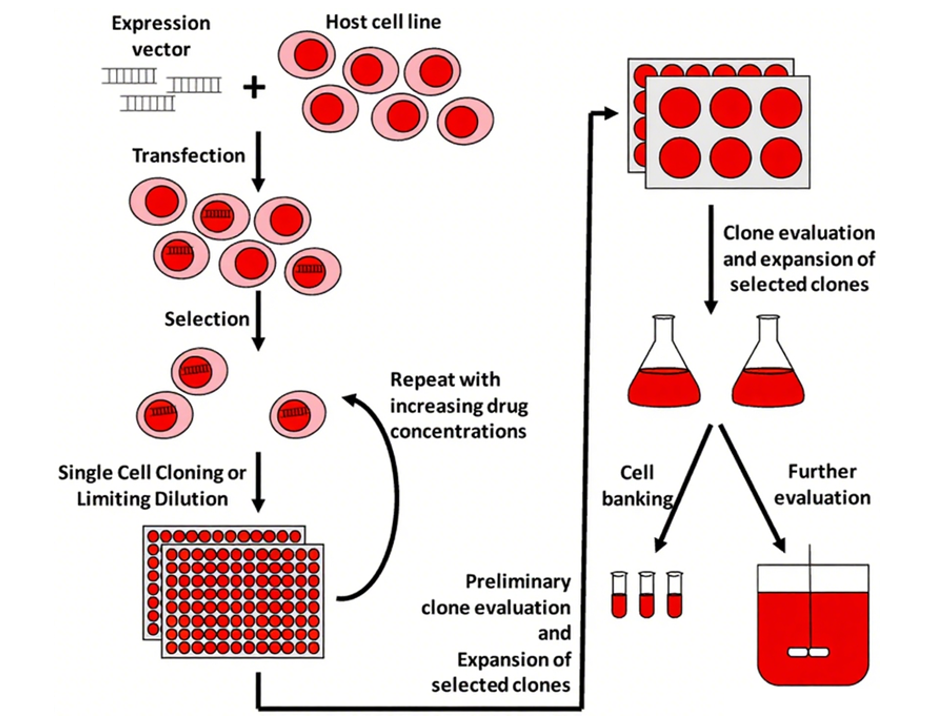
Challenges and Considerations
High Cost
The maintenance of mammalian cell cultures is expensive due to the complex media requirements and stringent culture conditions. This high cost can be a significant barrier to their widespread use, particularly in resource-limited settings.
Low Protein Yield
Compared to bacterial systems, mammalian systems typically yield lower amounts of recombinant protein. This low yield can increase production costs and limit the scalability of protein production.
Technical Complexity
The setup and optimization of mammalian cell cultures require specialized expertise and infrastructure. The complex bioprocesses involved in maintaining mammalian cell cultures demand skilled personnel and advanced facilities.
Conclusion
Mammalian cell expression systems hold great promise for the future of therapeutic protein production. Their unique ability to generate proteins with human-like post-translational modifications (PTMs) makes them indispensable for developing complex biopharmaceuticals. Ongoing research focuses on enhancing protein yield and reducing production costs through advanced genetic engineering and bioprocess optimization. This will likely expand their applications in personalized medicine and gene therapy, where precise protein modifications are critical. Additionally, advancements in synthetic biology and systems biology approaches are expected to further refine the control and efficiency of protein expression in mammalian systems, potentially revolutionizing the biopharmaceutical landscape.
References
1. Gray, D. Overview of Protein Expression by Mammalian Cells. Current Protocols in Protein Science, 1997,10(1), 591-5918.
2. Wendy Fitzgerald,Jean‐Charles Grivel. A universal nanoparticle cell secretion capture assay[J].Cytometry.2012(2)
3. Malm, M., Kuo, C.. Harnessing secretory pathway differences between HEK293 and CHO to rescue production of difficult to express proteins. Metabolic Engineering,2022, 72, 171-187.
4. Steven C.L.Ho,Esther Y.C.Koh. Control of IgG LC:HC ratio in stably transfected CHO cells and study of the impact on expression,aggregation,glycosylation and conformational stability.Journal of Biotechnology.2013(3-4)
5. Ju Hyun Park,Zesong Wang. Enhancement of recombinant human EPO production and glycosylation in serum-free suspension culture of CHO cells through expression and supplementation of 30Kc19.Applied Microbiology and Biotechnology.2012(3)
6. Kyoung Ho Lee,Masayoshi Onitsuka.Rapid construction of transgene-amplified CHO cell lines by cell cycle checkpoint engineering[J].Applied Microbiology and Biotechnology.2013(13)
7. Haldankar, R., Li, D., Saremi, Z. et al. Serum-free suspensin large-scale transient transfection of CHO cells in WAVE bioreactors. Mol Biotechnol 34, 191–199 (2006).
8. Khan, K. H. Gene Expression in Mammalian Cells and its Applications. Advanced Pharmaceutical Bulletin, 2013,3(2), 257-263.

