Western Blot Protocol
I. Sample Lysis
Adherent Cell Culture Lysate Preparation
- Place cell culture dishes on ice and wash cells with cold PBS.
- Remove PBS and add cold lysis buffer (1 mL per 10⁷ cells/100 mm dish).
- Use a pre-chilled plastic cell scraper to scrape adherent cells from the dish. Gently transfer the cell suspension to a pre-chilled centrifuge tube. Alternatively, digest cells with trypsin, wash with PBS, and resuspend cells in lysis buffer in the centrifuge tube.
- Shake continuously at 4 °C for 30 minutes.
- Centrifuge at 12,000 rpm for 5 minutes at 4 °C.
- Carefully remove the centrifuge tube from the centrifuge and place it on ice. Transfer the supernatant to a new pre-chilled tube on ice.
Suspension Cell Culture Lysate Preparation
- Centrifuge suspension cells at 300 g for 5 minutes, discard the supernatant, and resuspend cells in 10 mL cold PBS. Centrifuge again and discard PBS.
- Resuspend cells in residual PBS and add cold lysis buffer (1 mL per 10⁷ cells/100 mm dish). Shake continuously at 4 °C for 30 minutes.
- Centrifuge at 12,000 rpm for 5 minutes at 4 °C.
- Carefully remove the centrifure tube from the centrifuge and place it on ice. Transfer the supernatant to a new pre-chilled tube on ice.
Tissue Lysate Preparation
- Excise target tissue with clean instruments as quickly as possible on ice to prevent proteolysis.
- Place tissue in a mortar, pour in liquid nitrogen to "flash freeze," and immediately homogenize. Add lysis buffer and shake continuously at 4 °C for 2 hours. The lysis buffer volume should be determined based on the total tissue amount. The protein extract should not be overly diluted to prevent protein loss. Minimize sample volume for gel loading. The minimum concentration is 0.1 mg/mL, and the optimal concentration is 1-5 mg/mL.
- Centrifuge at 12,000 rpm for 20 minutes at 4 °C. Carefully remove the centrifuge tube from the centrifuge and place it on ice. Transfer the supernatant to a new pre-chilled tube on ice.
II. Sample Preparation
- Take a small amount of lysate for protein quantification. Determine the protein concentration of each cell lysate.
- Determine the protein loading amount and add 1/4 volume of 5X sample buffer.
- For sample reduction and denaturation, boil the mixed sample buffer at 100 °C for 5 minutes.
III. Loading and Gel Running
- Load the prepared samples into SDS-PAGE gel wells. The total protein loading amount for cell lysates or tissue homogenates is 20-30 μg, and for purified proteins, it is 10-100 ng.
- Run the gel at 100 V for 1-2 hours. Time and voltage may need optimization. We recommend following the manufacturer's instructions. Unless recommended otherwise by the antibody datasheet, use a reducing gel.
Gel Percentage by Protein Size
| Protein Molecular Weight | Separating Gel Concentration |
|---|---|
| <15 kDa | 20% |
| 15–40 kDa | 15% |
| 40-100 kDa | 10% |
| >100 kDa | 6-8% |
Gradient gels can also be used.
IV. Protein Transfer from Gel to Membrane
The membrane can be nitrocellulose or PVDF. PVDF membranes are commonly used and need to be activated with methanol for 1 minute before use and rinsed with transfer buffer before preparing the transfer stack. Transfer time and voltage need to be optimized according to the transfer buffer, and we recommend following the manufacturer's instructions. Protein transfer can be checked with Ponceau S staining before the blocking step.
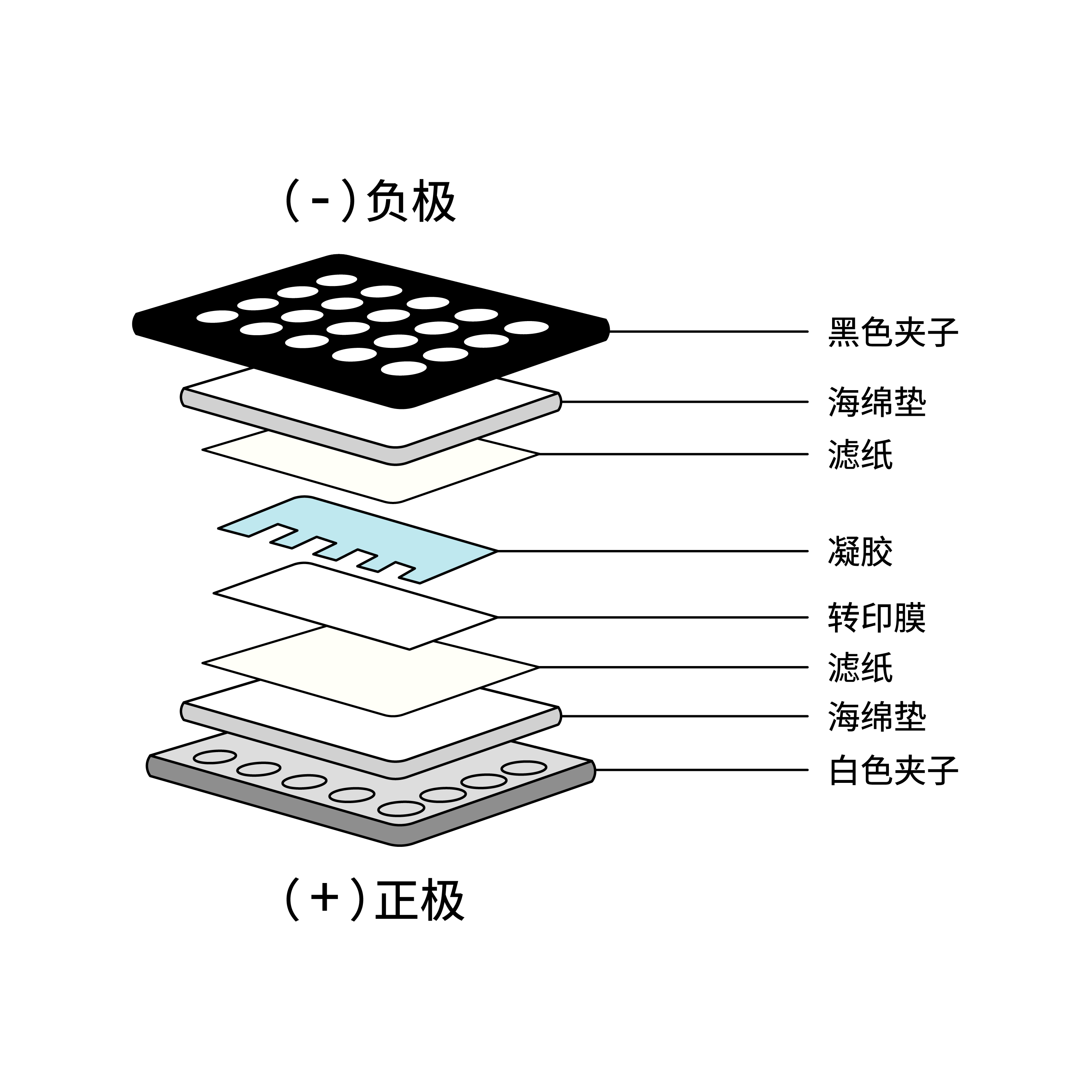
V. Blocking and Antibody Incubation
- Block the membrane with blocking buffer (5% skim milk/1% BSA) for 1 hour at room temperature or overnight at 4 °C.
- Remove the blocking solution and dilute the primary antibody with antibody dilution buffer according to the manufacturer's instructions. Incubate the membrane overnight at 4 °C or for 2 hours at room temperature.
- Wash the membrane with TBST three times, 5 minutes each.
- Dilute the conjugated secondary antibody with antibody dilution buffer according to the manufacturer's instructions and incubate the membrane for 1 hour at room temperature.
- Wash the membrane with TBST three times, 5 minutes each.
VI. Detection (ECL Method)
- ECL Working Solution Preparation: Mix equal volumes of Solution A and Solution B.
- Incubation: Evenly cover the membrane surface and incubate at room temperature in the dark for 1 minute.
- Remove excess liquid: (do not let the membrane dry).
- Exposure: Expose to X-ray film in a darkroom (5 seconds to 10 minutes).
VII. Buffer Recipes
RIPA Lysis Buffer
50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS. Add 1X protease inhibitor cocktail before use.
Transfer Buffer
25 mM Tris-base, 190 mM glycine, 20% methanol (for PVDF membranes), 10% methanol (for nitrocellulose membranes).
TBST Washing Buffer
20 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Tween-20.

