14-3-3 Family of proteins
The 14-3-3 family of proteins are highly conserved acidic eukaryotic proteins (25-32 kDa) abundantly present in the body[1]. There are seven different 14-3-3 isoforms in mammals, namely the α/β, σ, θ, γ, ε, η and ζ/δ, each of them binding to several hundreds of different proteins through phosphorylation. It was found to modulate a wide array of cellular processes, such as cell signalling, transcription, cell differentiation, cell apoptosis, protein trafficking, innate immunity, and glucose metabolism, to name a few [2]. Its dysregulation has been linked to the onset of critical illnesses such as cancers, neurodegenerative diseases and viral infections[3].
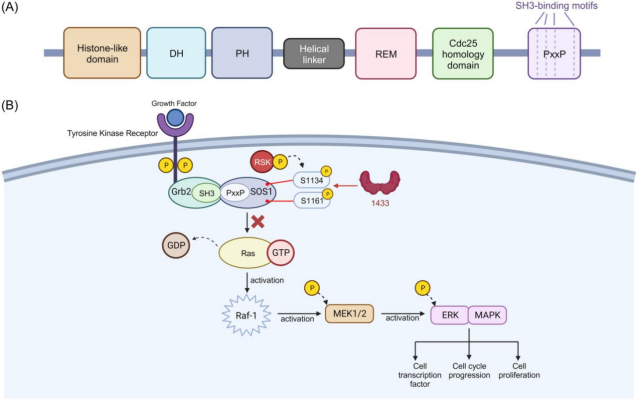
(A) The SOS1 domains (from N-C termini) with a Cdc25 homology domain (Ras-activator protein in yeast) attach a proline-rich sequence (PxxP). (B) The phosphorylation of S1134 and S1161 by RSK could facilitate the binding of 14-3-3 protein to SOS1, preventing Ras activation of the Ras-MAPK/ERK signalling pathway.
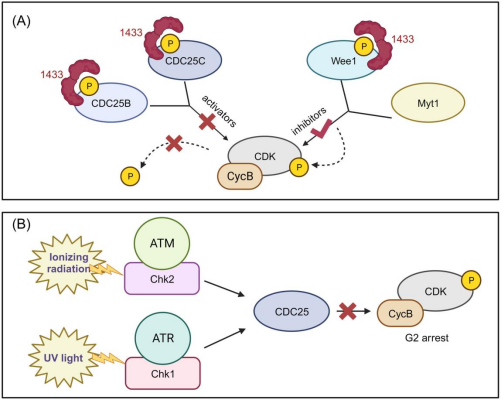
(A) The 14-3-3 protein binds to phosphorylated Cdc25B, Cdc25C and Wee1, inhibiting the activators and activating the inhibitors for CyclinB-Cdk1, respectively, shutting off the G2-M checkpoint, resulting in an inappropriate mitotic entry, a hallmark of cancer progression. (B) The CDC25s are self-regulated by Chk1 & 2 via the ATM and ATR pathway in response to re-entry into the mitosis or genotoxic stress induced by ionising radiation and ultraviolet light. Adapted from Gardino and Yaffe [4].
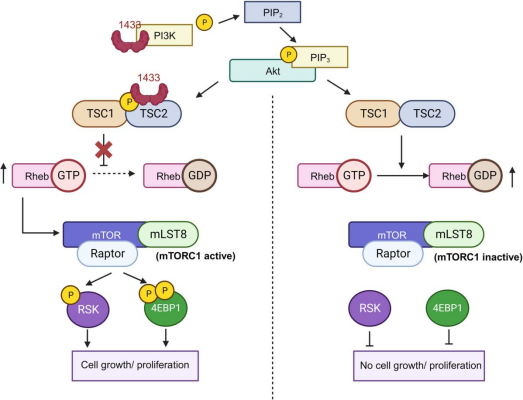
A general overview of 14-3-3 protein expressions in cancer. The 14-3-3 protein binds to the p85 regulatory unit of the PI3K, activating the PI3K-Akt pathway via Rheb, which in turn activates the mTOR (rapamycin) and its downstream targets, such as the 4EBP1 and RSK-MAPK signalling, leading to tumour proliferation. Adapted from Lipina and Hundal [5].
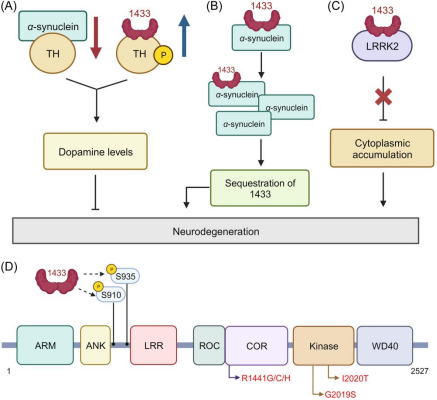
The role of 14-3-3 protein in Parkinson's disease. (A) 14-3-3 proteins interact with phosphorylated TH to prolong activation and prevent dephosphorylation of TH by pp2A (positive regulator). α-synuclein, on the other hand, binds to unphosphorylated TH to reduce its activity (negative regulator). (B) α-synuclein sequestrate 14-3-3 causes enhanced pro-apoptotic activity and neuronal toxicity, leading to neurodegeneration. (C) 14-3-3 protein binds to LRRK2 and protects neuronal cells from neurite shortening caused by LRRK2 cytoplasmic accumulation. (D) The LRRK2 domain contains the ankyrin-like repeats (ANK), Leucine-rich repeat (LRR), Ras of complex proteins (Roc), C-terminal of Roc (COR), kinase domain and WD40 domain. The R1441C/G/H, Y1699C and I2020T mutations in LRRK2 cause reduced S910 and S935 phosphorylation followed by reduced 14-3-3 binding, leading to the accumulation of LRRK2 in the cytoplasm, causing neurite dystrophy and neurotoxicity. Adapted from Shimada et al. [6].
Relevant antibodies
| Catalog# | Product Name | Reactivity | Application |
|---|---|---|---|
| AMRe01592 | 14-3-3 Rabbit Monoclonal Antibody | Human,Mouse,Rat | WB |
| AMRe06275 | 14-3-3 gamma (13M6) Rabbit Monoclonal Antibody | Human,Mouse,Rat | WB |
| APRab06283 | 14-3-3 ζ/δ Rabbit Polyclonal Antibody | Human,Mouse,Rat | WB,IHC-P,IF-P,IF-F,ICC/IF,ELISA |
| AMRe06274 | 14-3-3 epsilon (1W4) Rabbit Monoclonal Antibody | Human,Mouse,Rat | WB,ICC/IF,FC |
| AMRe01593 | 14-3-3 theta Rabbit Monoclonal Antibody | Human,Mouse,Rat | WB |
| APRab06284 | 14-3-3 η Rabbit Polyclonal Antibody | Human,Mouse,Rat | WB,IHC-P,IF-P,IF-F,ICC/IF,ELISA |
| APS0635 | HRP-conjugated Polyclonal Goat Anti-Rabbit IgG(H+L) Secondary Antibody | Rabbit | ELISA,WB,Dot blot |
| AMre80004 | GAPDH (12R9) Rabbit Monoclonal Antibody | Human,Mouse,Rat,Rabbit,Dog,Monkey | WB,ELISA |
Related Products
Super-sensitive ECL chemiluminescent reagent
References
- Low ZY, Yip AJW, Chan AML, Choo WS. 14-3-3 Family of Proteins: Biological Implications, Molecular Interactions, and Potential Intervention in Cancer, Virus and Neurodegeneration Disorders. J Cell Biochem. 2024 Jul;125(7):e30624. Epub 2024 Jun 30. [PMID: 38946063].
- Nathan KG, Lal SK. The Multifarious Role of 14-3-3 Family of Proteins in Viral Replication. Viruses. 2020 Apr 13;12(4):436. [PMID: 32294919].
- Obsilova V, Obsil T. Structural insights into the functional roles of 14-3-3 proteins. Front Mol Biosci. 2022 Sep 16;9:1016071. 2022.1016071. [PMID: 36188227]
- Gardino AK, Yaffe MB. 14-3-3 proteins as signaling integration points for cell cycle control and apoptosis. Semin Cell Dev Biol. 2011 Sep;22(7):688-95. Epub 2011 Sep 14. [PMID: 2194564].
- Zou Z, Tao T, Li H, Zhu X. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci. 2020 Mar 10;10:31. [PMID: 32175074].
- Shimada T, Fournier AE, Yamagata K. Neuroprotective function of 14-3-3 proteins in neurodegeneration. Biomed Res Int. 2013;2013:564534. Epub 2013 Dec 2. [PMID: 24364034].

