I. Core Principle
ELISA, which stands for Enzyme-Linked Immunosorbent Assay, is a foundational technique in biomedical research and diagnostics. Its core principle leverages the highly specific binding reaction between an antigen and its antibody, amplifying and visualizing this interaction through an enzymatic colorimetric reaction. This allows for the qualitative or quantitative detection of extremely trace amounts of a target substance (antigen or antibody).
Think of it as a "specific capture + chemical signal amplification" detection system. It primarily consists of three key components:
Solid Phase Carrier: Typically a 96- or 384-well polystyrene plate, used to adsorb ("coat") the antigen or antibody.
Immunological Reaction: The highly specific antigen-antibody binding reaction ensures the accuracy of the detection.
Enzymatic Signal Amplification: An enzyme (such as Horseradish Peroxidase-HRP or Alkaline Phosphatase-AP) conjugated to an antibody catalyzes a substrate to produce a color, fluorescent, or chemiluminescent signal. This transforms a weak immune reaction into a powerful, detectable signal.
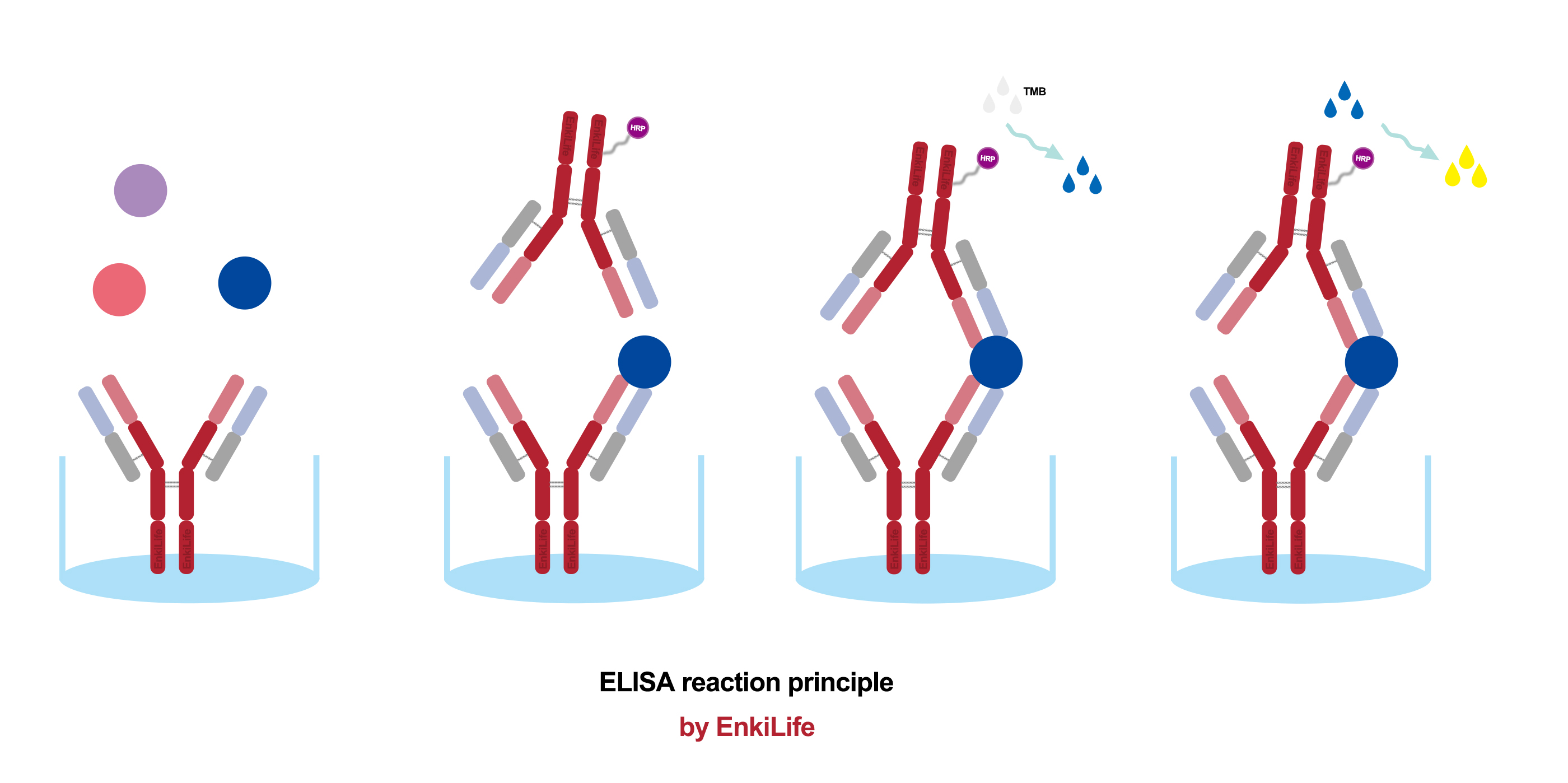
II. Main Types and Procedure (Using the classic "Sandwich" ELISA as an example)
There are several types of ELISA suited for different scenarios, with the Sandwich ELISA for antigen detection being the most common.
Experimental Procedure:
Coating: A specific capture antibody is immobilized onto the walls of the microplate wells. After incubation, any unbound antibody is washed away.
Blocking: Unrelated proteins (e.g., Bovine Serum Albumin-BSA or skimmed milk) are added to fill any remaining binding sites on the plate. This prevents the non-specific adsorption of proteins in subsequent steps, reducing background noise.
Sample Addition: The test sample (containing the antigen at an unknown concentration) or standards (samples with known antigen concentrations) are added and incubated. The target antigen in the sample is specifically "captured" and bound by the immobilized antibody. A wash step removes unbound material.
Detection Antibody Addition: A enzyme-linked antibody (conjugated antibody) specific to a different epitope on the same antigen is added and incubated. This antibody also binds to the captured antigen, forming an "antibody-antigen-enzyme-linked antibody" complex, hence the name "sandwich." Another wash follows.
Substrate Addition: A colorless substrate for the enzyme (e.g., TMB for HRP) is added. The enzyme catalyzes a reaction that converts the substrate into a colored product.
Stop & Measure: A stop solution (e.g., sulfuric acid) is added to halt the enzymatic reaction, fixing the color intensity. A microplate reader measures the Optical Density (OD value) of each well at a specific wavelength (e.g., 450 nm for TMB).
Data Analysis: A standard curve is plotted (Standard concentration vs. OD value). The concentration of the antigen in the unknown samples is calculated by interpolating their OD values from this standard curve.
Other Main Types:
Indirect ELISA: Primarily used to detect antibodies (e.g., antibodies produced after a pathogen infection, autoantibodies). Procedure: Coat antigen → Add test serum (contains primary antibody) → Add enzyme-linked secondary antibody (anti-primary antibody) → Develop color.
Competitive ELISA: Suitable for small molecule antigens (e.g., hormones, drugs). The antigen in the test sample competes with a labeled antigen for binding to a limited amount of antibody. The color intensity is inversely proportional to the concentration of the antigen in the sample.
Direct ELISA: The simplest procedure, but offers lower sensitivity and specificity. The enzyme-linked primary antibody reacts directly with the coated antigen.
III. Development History
The development of ELISA technology is built upon several Nobel Prize-worthy discoveries.
1940s-50s: Theoretical Foundation: Advances in immunology established the specificity of antigen-antibody reactions. Yalow and Berson invented the Radioimmunoassay (RIA) (Nobel Prize in 1977), which first combined immunoassays with highly sensitive detection, albeit using radioactive isotopes with associated safety hazards.
1971: Birth: Swedish scientists Eva Engvall and Peter Perlmann, and independently, Dutch scientists Anton Schuurs and Bea van Weemen, published methods almost simultaneously for using enzymes instead of radioisotopes for immunoassays. They were the first to name it "ELISA," marking the beginning of the modern ELISA technique.
1970s-80s: Commercialization and Proliferation: The advent of monoclonal antibody technology (Nobel Prize in 1984) drastically improved the specificity and reproducibility of ELISA. Major biotech companies began developing commercial ELISA kits, rapidly transforming it from a lab method into a standard tool for clinical diagnostics and research.
Present: Optimization and Innovation: The technology continues to be refined: higher affinity antibodies, more stable enzymes, more sensitive substrates (e.g., chemiluminescence - ECL), and automated equipment (fully automated plate washers and readers) have continuously expanded ELISA's sensitivity, throughput, and application range.
IV. Technical Advantages
High Sensitivity:
Capable of detecting extremely trace amounts of substance at picogram(pg/mL) or even femtogram (fg/mL) levels, thanks to the efficient amplification effect of the enzymatic reaction.High Specificity:
Based on the antigen-antibody reaction, especially when using monoclonal antibodies, it can accurately distinguish between structurally similar molecules, offering strong resistance to interference.High Throughput:
The 96-well or 384-well plate format allows for the simultaneous processing of a large number of samples, is easily automated, and is ideal for large-scale screening and epidemiological studies.Quantitative Analysis:
The use of a standard curve enables the precise determination of the target's concentration, not just its presence or absence.Simple Operation and Ease of Standardization:
The procedure is well-established and requires relatively low technical skill from the operator. Commercial kits provide pre-coated plates and optimized reagents, making results comparable across different laboratories.High Safety:
Unlike RIA, it does not use radioactive isotopes, eliminating the need for special protective measures and waste disposal procedures.Cost-Effective:
Instrumentation (microplate readers) is relatively inexpensive, reagent costs are manageable, and the cost per test is low.
V. Application Fields
Clinical Diagnostics: Detection of viral/bacterial infections (HIV, HBV, COVID-19 antibodies/antigens), hormone level testing (hCG for pregnancy tests), tumor marker screening, autoimmune disease diagnosis, allergen detection.
Biomedical Research: Analysis of protein expression levels, signal transduction pathway studies, identification of protein-protein interactions, vaccine development and evaluation.
Food Safety: Detection of toxins (e.g., aflatoxins), pathogenic microorganisms (e.g., Salmonella), antibiotic residues in food.
Agriculture and Animal/Plant Quarantine: Detection of plant viruses, veterinary drug residues, etc.
Limitations:
Developing a new ELISA assay can be time-consuming; it primarily detects soluble proteins; potential for cross-reactivity exists; and its dynamic range is relatively narrow compared to some newer techniques.
In summary, since its inception, ELISA technology has become a "gold standard" and core technology in the field of immunoassay due to its exceptional sensitivity, specificity, high throughput, and ease of use. It plays an indispensable role in both scientific research and clinical practice.
