Human endometrial cells (EEC) refer to epithelial cells derived from the human endometrium, which have important application value in scientific research. EEC can be divided into normal endometrial cells and endometrial cancer cells (such as endometrial adenocarcinoma, EEC).
1. Normal endometrial cells: These cells are typically used to study the physiological functions and pathological changes of the endometrium. For example, through single-cell sequencing technology, research has found that EEC may originate from endometrial epithelial cells rather than stromal cells, providing a new perspective for understanding the origin and pathological processes of the endometrium. In addition, normal EECs can be cultured in vitro under specific culture conditions, such as using DMEM/Ham's F12 medium, and their phenotype can be confirmed by immunohistochemistry [2].
2. Endometrial cancer cells (EEC): Endometrial cancer is the most common type of malignant tumor of the endometrium, accounting for approximately 75% of all endometrial cancers. The pathogenesis of EEC is complex and closely related to the expression of estrogen receptor (ER) and progesterone receptor (PR). Research has found that the molecular characteristics of EEC include PTEN gene mutations, PIK3CA mutations, etc. [1]. In addition, the invasiveness and prognosis of EEC are associated with multiple molecular markers, such as p53 mutations and POLE hypermutation status [14].
3. Cell culture and application: EEC requires specific conditions for in vitro culture, such as recommending the use of MEM medium and adding fetal bovine serum (FBS) and antibiotics. These cells can be used to study the physiological and pathological status of the endometrium, as well as for drug screening and the establishment of disease models [2].
4. Research progress: In recent years, single-cell sequencing technology has played an important role in EEC research, revealing the origin and pathological process of EEC. For example, research has found that EEC originates from non porous glandular epithelial cells and undergoes significant changes in the stromal microenvironment and immune environment during the progression of endometrial cancer.
The human endometrial cells (EEC) have significant importance in both basic research and clinical applications, as their research contributes to a deeper understanding of the physiological functions, pathological mechanisms, and diagnosis and treatment of related diseases of the endometrium.
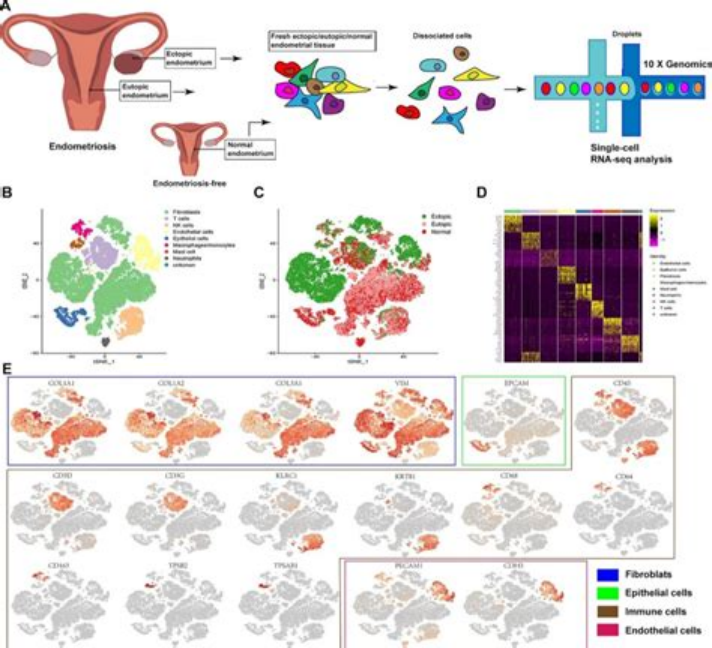
The research progress of single-cell sequencing technology for human endometrial cells (EEC) mainly focuses on the following aspects:
1. Single cell transcriptome sequencing reveals the origin and pathological process of EEC:
In October 2022, Ren Xiaojun and others from Fudan University established a single-cell atlas of endometrial adenocarcinoma (EEC) patients through single-cell transcriptome sequencing (scRNA seq), revealing changes in cell status during EEC development. The study found that EEC originates from endometrial epithelial cells (non ciliated glandular epithelium) rather than stromal cells, and identified LCN2+/SAA1/2+cells as a characteristic subgroup of endometrial tumor development [15].
2. Application of single-cell sequencing technology in female reproductive health:
Endometrial single-cell sequencing technology has gradually become an important tool for studying female reproductive health. This technology analyzes the gene expression of individual cells in endometrial tissue through high-throughput sequencing, revealing the biological characteristics and functions of the endometrium, providing a new approach for infertility diagnosis, early diagnosis of endometrial cancer, research on endometrial physiological and pathological mechanisms, and personalized treatment plan development.
3. Application of single-cell sequencing technology in menstrual cycle:
The study published on December 18, 2024 revealed the single-cell profile and physiological mechanisms of the endometrium during the menstrual cycle. Although this study has some limitations, such as the need for a large number of cell samples and the inability to dynamically observe cellular changes, it provides a solid foundation for future research and clinical applications.
4. Single cell analysis reveals the characteristics of endometrial stem cells:
On June 22, 2023, a study revealed the characteristics of epithelial stem cells (EESCs) in human endometrial glands through single-cell analysis, providing a new platform for studying EESC function and endometrial gland development. The study found that the endometrial glandular model has a morphology and marker expression pattern similar to that of human endometrial glands, and identified potential eeSC marker genes [16].
5. Application of single-cell sequencing technology in the study of gynecological malignant tumors:
Single cell sequencing technology has important applications in the study of the mechanisms and heterogeneity of gynecological malignant tumors. For example, analyzing the tumor microenvironment of endometrial cancer through single-cell RNA sequencing revealed the impact of cell-cell and cell-matrix interactions within the tumor microenvironment on EC progression [17].
6. Application of single-cell sequencing technology in endometrial receptivity:
Single cell sequencing technology has significant advantages in analyzing the differences between different cells in the endometrium. For example, through single-cell transcriptome analysis, the menstrual cycle can be divided into four main stages and the "implantation window" of the embryo can be more accurately determined [37].
7. Construction of single-cell reference map:
A study published on September 20, 2024, constructed a high-resolution Human Endometrial Cell Atlas (HECA) that integrated single-cell transcriptome data from 63 women (including endometriosis patients and non patients) and identified previously unreported cell types.
What are the specific roles of PTEN gene mutations and PIK3CA mutations in endometrial cancer cells (EEC)?
In endometrial cancer cells (EEC), mutations in PTEN and PIK3CA genes play a crucial role in the occurrence and development of tumors.
1. PTEN gene mutation:
The oPTEN gene is a tumor suppressor gene whose main function is to regulate cell growth and proliferation by inhibiting the PI3K-AKT/mTOR signaling pathway. Mutations or deletions of PTEN can lead to excessive activation of this pathway, thereby promoting the occurrence and development of tumors [20].
In endometrial cancer, the mutation rate of PTEN gene is relatively high, especially in type I endometrial cancer, where the mutation rate can reach 64-80% [20]. PTEN mutations typically manifest as inactive variants, such as truncation mutations or negative substitutions involving R130 [19].
The oPTEN mutation is closely related to the early occurrence of endometrial cancer and is considered one of the earliest events in the progression of endometrioid carcinoma [22].
2. PIK3CA gene mutation:
The oPIK3CA gene encodes the catalytic subunit p110 α of PI3K, and its mutation leads to sustained activation of the PI3K/AKT/mTOR signaling pathway. This activated state can promote cell growth, proliferation, and survival [21].
In endometrial cancer, the mutation rate of PIK3CA gene is also relatively high, especially in type I endometrial cancer, where the mutation rate can reach 22-59% [20]. The PIK3CA mutations are mainly activating mutations, which enable the PI3K protein to maintain an activated state without relying on upstream signals [21].
The oPIK3CA mutation and PTEN mutation often occur together and are mutually exclusive. In tumors containing PIK3R1 mutations, AKT-T308 phosphorylation levels are higher and lowest in PTEN wild-type (WT) tumors [18].
In summary, mutations in the PTEN gene result in the loss of inhibitory effects on the PI3K-AKT/mTOR signaling pathway, while mutations in the PIK3CA gene directly activate this pathway.
How to confirm the phenotype of normal endometrial cells through immunohistochemistry?
The confirmation of the phenotype of normal endometrial cells through immunohistochemistry can refer to the following steps:
1. Sample preparation:
Fix the endometrial tissue sample (e.g. fix with 4% paraformaldehyde for half an hour) [23].
Prepare paraffin embedded sections with a thickness of 4 μ m [24].
2. Antigen repair:
Treat the slices with Triton X-100 for 20 minutes to enhance antigen exposure [23].
Perform high-pressure antigen repair to ensure effective binding of antibodies to antigens [24].
3. Blocking endogenous peroxidase:
Use a 0.3% H2O2 solution to block endogenous peroxidase and prevent non-specific reactions [23].
4. Primary antibody incubation:
Add rabbit anti human Survivor as primary antibody and incubate for 2 hours [23].
For other markers such as CK and Vim, mouse anti human cell CK antibody (1:200) and rabbit anti human cell Vim antibody (1:200) can be used for incubation [25].
5. Secondary antibody incubation:
Add HRP labeled secondary antibody and incubate in a 37 ℃ water bath for 30 minutes [24].
6. Color reaction:
Use DAB as a substrate for color reaction, observe the color reaction and stop it in a timely manner [23, 24].
Observe the color reaction under a microscope and perform hematoxylin counterstaining [24].
7. Result determination:
Score based on staining intensity and the proportion of positive cells [24].
For the expression of CK and Vim, CK is positively stained in the cytoplasm of normal endometrial glandular epithelial cells, while Vim expression is negative [25].
By following the above steps, the phenotype of normal endometrial cells can be effectively confirmed. Specifically, positive expression of CK indicates that the cells are epithelial cells, while negative expression of Vim further confirms that these cells are glandular epithelial cells [25].
What molecular markers are associated with the invasiveness and prognosis of endometrial cancer cells (EEC)?
The invasiveness and prognosis of endometrial cancer cells (EEC) are associated with multiple molecular markers. Here are some key molecular markers and their roles:
1. POLE mutation: POLE mutation is relatively rare in EEC, but is associated with good prognosis. All EECs with POLE mutations showed similar prognosis to other POLE mutated endometrial cancers [26].
2. MMR deficiency: Microsatellite instability (MSI) high mutation (MMRd) is an important molecular subtype in EEC, associated with poor prognosis [29].
3. p53 abnormality: p53 abnormality is also common in EEC and is associated with poor prognosis. About one-third of EECs belong to the p53 abnormal category [26].
4. UBE2C: UBE2C is a member of the ubiquitin ligase E2 family, significantly overexpressed in EEC and associated with poor prognosis. The high expression of UBE2C may be related to gene mutations, amplification, and DNA hypomethylation, and its interference can inhibit the migration and invasion of EEC cells [27].
5. HABP1: The high expression of HABP1 in EEC is closely related to advanced EC, deep muscle infiltration, lymphatic vessel invasion, lymph node metastasis, and recurrence. Moreover, the overall survival (OS) and disease-free survival (DFS) of high expression individuals are significantly shortened, which is an independent factor affecting prognosis [28].
6. CRABP2: CRABP2 is a cell retinoic acid binding protein that is overexpressed in various cancers and participates in the retinoic acid signaling pathway, promoting cell proliferation, differentiation, and apoptosis. The high expression of CRABP2 is associated with a high risk of endometrial cancer [31].
7. TRAP1 and CAMSAP3: In early endometrial cancer, the expression of TRAP1 and CAMSAP3 is significantly downregulated, and these proteins exhibit more invasiveness and poor prognosis in specific cell clusters. The predictive model constructed with TP53 can improve the diagnostic efficiency of early EC patients [32].
8. PTEN, ARID1A, PIK3CA: Mutations or abnormal expression of these genes are common in EEC and closely related to the occurrence, development, and prognosis of tumors [29,33].
9. CAFs: Tumor associated fibroblasts (CAFs) promote tumor invasion and metastasis in EEC by inducing epithelial mesenchymal transition (EMT). Overexpression of PTTG gene in CAFs group resulted in downregulation of EMT expression markers and decreased invasion and metastasis ability in EEC cells after inhibition of PTTG expression [30].
What are the application cases of endometrial cells (EEC) in drug screening and disease model establishment?
The application cases of endometrial cells (EEC) in drug screening and disease model establishment include the following aspects:
1. Development of a three-dimensional endometrial cell model:
The Kevin Osteen team has developed a three-dimensional endometrial cell model (EndoChip) that mimics the structure of human endometrium, including different cell types and vascular systems. This model can be used for efficient contraceptive screening, by validating existing drugs to discover new contraceptives with minimal adverse effects [34].
2. Establishment of hEM3 cell line:
The research team at Johns Hopkins University has established a new human endometrial epithelial cell line hEM3, which can stably grow in vitro without exhibiting aging. The hEM3 cell line expresses Pitt specific protein markers on the endometrium and is suitable for gene editing. Researchers have specifically knocked out and cloned the ARID1A gene, and found that both HDAC inhibitors and PARP inhibitors can effectively target cells with ARID1A deficiency. This provides a new tool for the pathophysiological research and development of effective and precise therapies for endometrial related diseases [35].
3. Application of EDS cells:
OEDS cells (embryonic stem cells) have broad application prospects in medical research, including disease model establishment, tissue engineering, and drug screening. For example, EDS cells can be used to establish a disease model of endometriosis, which helps to study the pathogenesis of the disease and find new treatment methods. In addition, EDS cells can also be used to screen and study drugs for the treatment of endometriosis, accelerating the development process of new drugs.
4. Establishment of HEM15a cell line:
The oHEM15a cell line is derived from in situ endometrial stromal cells of patients with endometriosis and established after immortalization treatment. This cell line has the characteristic of adherent growth, expressing estrogen receptor (ER) and progesterone receptor (PR), and is suitable for studying the pathological mechanism and treatment methods of endometriosis. HEM15a cells maintain genomic stability in multiple passages, providing an important tool for disease models and drug screening.
5. Construction of rat endometrial organoids:
The research team from Shantou University utilized rat endometrial epithelial stem cells to generate uterine organoids and conducted identification. These organoids not only exhibit long-term stable proliferation ability, but also maintain the glandular structure, cell polarity, and various functional characteristics of the endometrial epithelium. This model provides valuable insights into the pathogenesis and pathophysiology of endometrial diseases, as well as an efficient platform for drug screening and efficacy evaluation [36].
References
2. A 3D endometrial organotypic model simulating the acute inflammatory decidualisation initiation phase with epithelial induction of the key endometrial receptivity marker, integrin αVβ3.[ PMID: 34532597]
4. Mining TCGA Data for Key Biomarkers Related to Immune Microenvironment in Endometrial cancer by Immune Score and Weighted Correlation Network Analysis.[PMID: 33869285]
5. An IGF1-expressing endometrial stromal cell population is associated with human Decidualization. [PMID: 36482461]
6. Proteomic analysis identifies interleukin 11 regulated plasma membrane proteins in human endometrial epithelial cells in vitro. [PMID: 21619711]
7. TLR9 and RIG-I signaling in human endocervical epithelial cells modulates inflammatory responses of macrophages and dendritic cells in vitro. [PMID: 24409285]
9. The expression and functionality of stromal caveolin 1 in human adenomyosis. [PMID: 23442759]
10. Increased epithelial stem cell traits in advanced endometrial endometrioid carcinoma.[PMID: 20015385]
11. Angiotensin-(1-7) and angiotensin Ⅱ induce the transdifferentiation of human endometrial epithelial cells in vitro. [PMID: 24718590]
12. Protease secretions by the invading blastocyst induce calcium oscillations in endometrial epithelial cells via the protease-activated receptor 2. [PMID: 37060079]
13. miR 22 inhibits proliferation and invasion in estrogen receptor α positive endometrial endometrioid carcinomas cells. [PMID: 34278458]
14. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists. [PMID: 37627129]
15. Single-cell transcriptome sequencing reveals the origin and pathogenesis of endometrial adenocarcinoma. Ren Xiaojun et al., Fudan University.[2022-10]
16. P-797 Single-cell analysis sheds light on investigation of epithelial stem/progenitor cells in human endometrial organoid. J Li等.
17. Single-cell sequencing revealed the heterogeneity and intratumoral interference of endometrial carcinoma in humans. Zhicheng Yu et al..[2022-05-13]
18. Proteogenomic insights suggest druggable pathways in endometrial carcinoma. Dou Y, et al. 2023.
19. Mutational profile of endometrial hyperplasia and risk of progression to endometrioid adenocarcinoma. [PMID: 32187665]
20. CHD4 R975H mutant activates tumorigenic pathways and promotes stemness and M2-like macrophage polarization in endometrial cancer.[PMID: 39127769]
21. PI3 The K pathway and its mechanism of action in tumor. Master's and doctoral Experiment Center.[2022-11-02]
22. Current status of clinical diagnosis and treatment of endometrial carcinoma. Zhang Kun, Guo Hongyan. [2017-01-24]
23. Regulation of survival and apoptosis of endometrial adenocarcinoma cells by normal endometrial stromal cells. Shi Min et al.
24. Liu Xuying, Zou Cunhua et al. Expression and significance of SIRT-1 and SUMO-1 in type I endometrial carcinoma [J]. Clinical Medical Progress, 2021, 11(10): 4437-4444.
25. Optimized isolation and culture of normal endometrial primary glandular epithelial cells. Lu Wen et al.
26. Molecular Classification of Endometrial Cancer and the 2023 FIGO Staging: Exploring the Challenges and Opportunities for Pathologists.[PMID: 37627129]
27. UBE2C serves as a prognosis biomarker of uterine corpus endometrial carcinoma via promoting tumor migration and invasion.[ PMID: 37803076]
28. Li Ranhong et al. Research progress on tumor markers in the diagnosis and prognosis assessment of endometrial carcinoma [J/OL]. Chinese Journal of Maternal and Child Clinical Medicine (Electronic Edition), 2016, 12(4) : 484-488.
29. Comprehensive molecular characterization of early stage grade 3 endometrioid endometrial adenocarcinoma.[PMID: 39126895]
30. Study on the role and mechanism of tumor-associated fibroblasts in promoting endometrial cancer cell mesenchymal transition. Zhejiang University, etc.[2022-08-17]
31. CRABP2 - A novel biomarker for high-risk endometrial cancer.[ PMID: 36163055]
32. Multi-omics profiling reveal cells with novel oncogenic cluster, TRAP1low/CAMSAP3low, emerge more aggressive behavior and poor-prognosis in early-stage endometrial cancer.[ PMID: 38880903]
33. Molecular and genetic prognostic factors that may influence fertility-preserving treatment (FST) decisions in patients with early endometrial carcinoma (ES-EC)[2022-08-30]
34. Development of a Novel Organ-On-Chip of the Endometrium. Kevin Osteen,etc.
35. A novel human endometrial epithelial cell line for modeling gynecological diseases and for drug screening.[ PMID: 34376780]
36. Generation and Characterization of Rat Uterus Organoids from Rat Endometrial Epithelial Stem Cells. [PMID: 39158276]
37.Advances in the study of multi-omics technology in human endometrial receptivity. Li Rong et al.
