Overview and Functions of Akt Protein
Akt (also known as PKB or Rac) plays a critical role in regulating cell survival and apoptosis[1]. This protein kinase can be activated by insulin, various growth factors, and survival factors, functioning through a wortmannin-dependent pathway involving PI3 kinase[2].
Activation of Akt requires phospholipid binding and phosphorylation at two sites: phosphorylation at the catalytic domain Thr308 mediated by PDK1[3], and phosphorylation at the carboxyl terminal Ser473. The previously unknown PDK2 responsible for phosphorylating Akt at Ser473 has been identified as mTOR (mammalian target of rapamycin), which exists in a rapamycin-insensitive complex containing rictor and Sin1[4].
Akt inhibits apoptosis and promotes cell survival by phosphorylating and inactivating multiple target proteins, including Bad[5], forkhead transcription factors, c-Raf, and caspase-9. PTEN phosphatase is a major negative regulator of the PI3K/Akt signaling pathway[6-9]; LY294002 is a specific PI3 kinase inhibitor[10].
Another important function of Akt is regulating glycogen synthesis by phosphorylating and inactivating GSK-3α and GSK-3β[10], and it may also be involved in insulin-stimulated glucose transport[11]. In addition to regulating survival and glycogen synthesis, Akt participates in cell cycle regulation through: 1) preventing GSK-3β-mediated phosphorylation and degradation of cyclin D1[12], and 2) negatively regulating cyclin-dependent kinase inhibitors p27 Kip1[14] and p21 Waf1/Cip1[15].
Akt also plays a key role in cell growth: it can directly phosphorylate mTOR in the rapamycin-sensitive complex containing raptor[16]; more importantly, Akt can phosphorylate and inactivate tuberous sclerosis protein 2 (TSC2/tuberin) — a negative regulator of mTOR in the mTOR-raptor complex[17,18].
Mechanisms of Akt Signaling Regulation
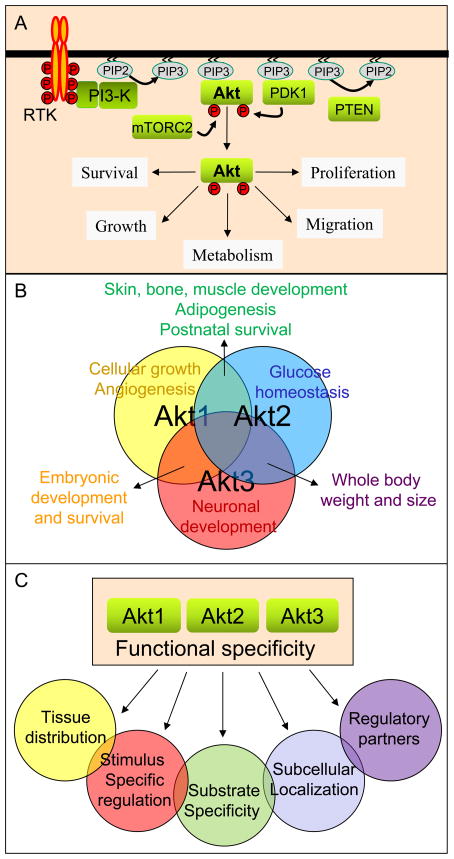
A. Growth factor-mediated Akt activation: In response to various growth factors and cytokines, Akt is activated downstream of PI3 kinase through a multi-step mechanism. Activated PI3 kinase converts PIP2 to PIP3, providing recruitment sites on the cell membrane for PH domain-containing proteins (including Akt and PDK1 kinases). Following translocation to the cell membrane, Akt's catalytic domain is phosphorylated by PDK1 and its hydrophobic motif by mTORC2, ultimately conferring catalytic activity. Activated Akt regulates multiple cellular functions by phosphorylating various downstream target proteins within the cell.
B. Overlapping and specific functions of Akt family members: Phenotypic analysis of single/double Akt isoform knockout mice has elucidated shared and unique functions of Akt isoforms, including:
- Akt1: Skin, bone, and muscle development, postnatal survival, cell growth, angiogenesis
- Akt2: Glucose homeostasis, adipogenesis
- Akt3: Neuronal development, overall body weight and size, embryonic development and survival
C. Potential mechanisms determining Akt isoform functional specificity: These involve tissue distribution, regulatory partners, stimulating signals, subcellular localization, specific regulation, and substrate specificity.
Specific Signaling of Akt2 in Insulin-Mediated Glucose Uptake
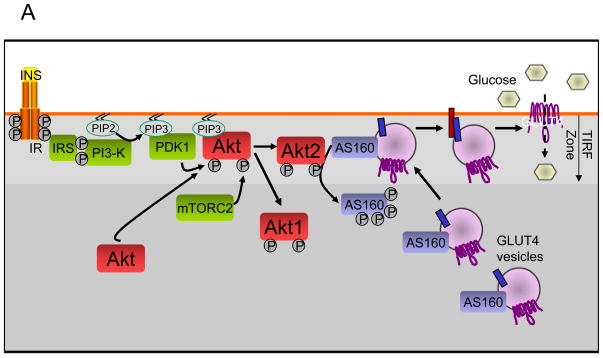
Following insulin binding to the insulin receptor (IR) on adipocyte surfaces, the receptor is activated and undergoes autophosphorylation, subsequently recruiting and phosphorylating insulin receptor substrate proteins (IRS). Phosphorylated IRS provides docking sites for PI3 kinase, which converts PIP2 to PIP3, thereby creating sites for Akt kinase recruitment to the cell membrane.
In response to PI3 kinase activation, both Akt1 and Akt2 translocate to the cell membrane and are activated through phosphorylation by PDK1 and mTORC2. Using Akt1/Akt2 reporter genes and total internal reflection fluorescence (TIRF) microscopy, it was observed that Akt2 accumulates to a greater extent than Akt1 in the membrane region (TIRF zone) following insulin stimulation. This unique subcellular distribution facilitates Akt2-mediated phosphorylation and inactivation of the Rab GAP protein AS160, which is likely located on GLUT4-containing vesicles; upon AS160 inactivation, GLUT4 vesicles can dock and fuse with the cell membrane, ultimately allowing glucose entry into the cell.
Relevant Antibodies
| Catalog# | Product Name | Reactivity | Application |
|---|---|---|---|
| APRab04209 | Akt (phospho Ser473) Rabbit Polyclonal Antibody | Human, Mouse, Rat, Chicken | IF-P, IF-F, ICC/IF, WB, IHC-P, ELISA |
| AMRe21259 | Akt (pan) Rabbit Monoclonal Antibody | Human, Mouse, Rat | WB, IHC, IF, IP, ELISA |
| AMM86142 | Phospho-Akt (S473) Mouse(7F9) Mouse Monoclonal Antibody | Human | WB, IHC |
| AMRe06740 | AKT1 (5O1) Rabbit Monoclonal Antibody | Human, Mouse | WB, IHC-P, ICC/IF, FC, IP, IF-P |
| APRab04219 | Akt2 (phospho Ser474) Rabbit Polyclonal Antibody | Human, Mouse, Rat | WB, IHC-P, IF-P, IF-F, ICC/IF, ELISA |
| AMM80810 | AKT2 Mouse Monoclonal Antibody | Human, Rat, Monkey | WB, IHC, ICC, ELISA |
| APS0635 | HRP-conjugated Polyclonal Goat Anti-Rabbit IgG(H+L) Secondary Antibody | Rabbit | ELISA, WB, Dot blot |
| APS0631 | HRP-conjugated Polyclonal Goat Anti-Mouse IgG(H+L) Secondary Antibody | Mouse | ELISA, WB, Dot blot |
| AMre80004 | GAPDH (12R9) Rabbit Monoclonal Antibody | Human, Mouse, Rat, Rabbit, Dog, Monkey | WB, ELISA |
Related Products
Super-sensitive ECL chemiluminescent reagent
References
- Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997 Feb 21;88(4):435-7. [PMID: 9038334].
- Burgering BM, Coffer PJ. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995 Aug 17;376(6541):599-602. [PMID: 7637810].
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996 Dec 2;15(23):6541-51. [PMID: 8978681].
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005 Feb 18;307(5712):1098-101. [PMID: 15718470].
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998 Nov 13;282(5392):1318-21. [PMID: 9812896].
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999 Mar 19;96(6):857-68. [PMID: 10102273].
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt (protein kinase B). Science. 1999 Nov 26;286(5445):1741-4. [PMID: 10576742].
- Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci U S A. 1999 Apr 13;96(8):4240-5. [PMID: 10200246].
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994 Feb 18;269(7):5241-8. [PMID: 8106507].
- Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)--a key regulator of glucose transport? FEBS Lett. 2001 Mar 16;492(3):199-203. [PMID: 11257494].
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995 Dec 21-28;378(6559):785-9. [PMID: 8524413].
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998 Nov 15;12(22):3499-511. [PMID: 9832503].
- Gesbert F, Sellers WR, Signoretti S, Loda M, Griffin JD. BCR/ABL regulates expression of the cyclin-dependent kinase inhibitor p27Kip1 through the phosphatidylinositol 3-Kinase/AKT pathway. J Biol Chem. 2000 Dec 15;275(50):39223-30. [PMID: 11010972].
- Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001 Mar;3(3):245-52. [PMID: 11231573].
- Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999 Dec 1;344 Pt 2(Pt 2):427-31. [PMID: 10567225].
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002 Sep;4(9):648-57. [PMID: 12172553].
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell. 2002 Jul;10(1):151-62. [PMID: 12150915].
- Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017 Apr 20;169(3):381-405. [PMID: 28431241].

Flora
Flora is a technical support expert at EnkiLife, familiar with immunology and neuroscience, dedicated to providing customers with high-quality product combinations and technical support to help achieve research in neurodegenerative diseases and other neuroscience areas.
