Size:100μL Price:$220
Size:200μL Price:$380
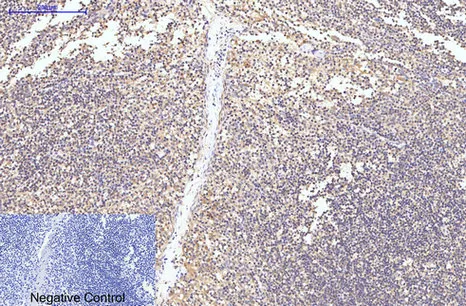
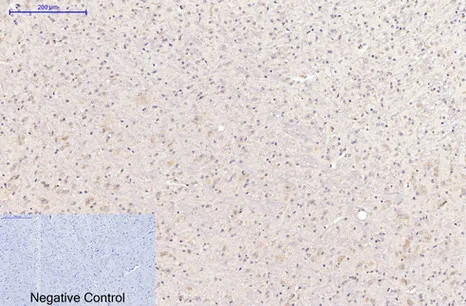
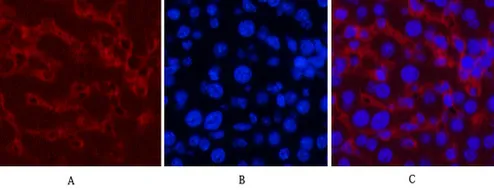
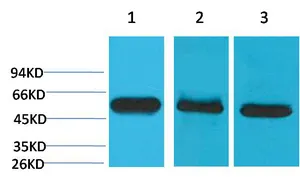
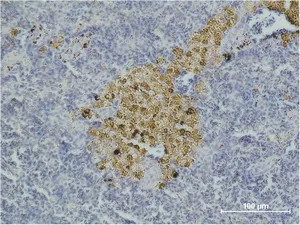
Application:WB,IHC,ICC/IF
Reactivity:Human,Mouse,Rat
Conjugate:Unconjugated
Optional conjugates: Biotin, FITC (free of charge). See other 26 conjugates.
Gene Name:CASP8
Summary
Performance
Immunogen
Application
Background
This gene encodes a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes composed of a prodomain, a large protease subunit, and a small protease subunit. Activation of caspases requires proteolytic processing at conserved internal aspartic residues to generate a heterodimeric enzyme consisting of the large and small subunits. This protein is involved in the programmed cell death induced by Fas and various apoptotic stimuli. The N-terminal FADD-like death effector domain of this protein suggests that it may interact with Fas-interacting protein FADD. This protein was detected in the insoluble fraction of the affected brain region from Huntington disease patients but not in those from normal controls, which implicated the role in neurodegenerative diseases. Many altcatalytic activity:Strict requirement for Asp at position P1 and has a preferred cleavage sequence of (Leu/Asp/Val)-Glu-Thr-Asp-|-(Gly/Ser/Ala).,disease:Defects in CASP8 are the cause of caspase-8 deficiency (CASP8D) [MIM:607271]. CASP8D is a disorder resembling autoimmune lymphoproliferative syndrome (ALPS). It is characterized by lymphadenopathy, splenomegaly, and defective CD95-induced apoptosis of peripheral blood lymphocytes (PBLs). It leads to defects in activation of T-lymphocytes, B-lymphocytes, and natural killer cells leading to immunodeficiency characterized by recurrent sinopulmonary and herpes simplex virus infections and poor responses to immunization.,domain:Isoform 9 contains a N-terminal extension that is required for interaction with the BCAP31 complex.,function:Most upstream protease of the activation cascade of caspases responsible for the TNFRSF6/FAS mediated and TNFRSF1A induced cell death. Binding to the adapter molecule FADD recruits it to either receptor. The resulting aggregate called death-inducing signaling complex (DISC) performs CASP8 proteolytic activation. The active dimeric enzyme is then liberated from the DISC and free to activate downstream apoptotic proteases. Proteolytic fragments of the N-terminal propeptide (termed CAP3, CAP5 and CAP6) are likely retained in the DISC. Cleaves and activates CASP3, CASP4, CASP6, CASP7, CASP9 and CASP10. May participate in the GZMB apoptotic pathways. Cleaves ADPRT. Hydrolyzes the small-molecule substrate, Ac-Asp-Glu-Val-Asp-|-AMC. Likely target for the cowpox virus CRMA death inhibitory protein. Isoforms 5, 6, 7 and 8 lack the catalytic site and may interfere with the pro-apoptotic activity of the complex.,online information:CASP8 mutation db,polymorphism:Genetic vaiations in CASP8 are associated with reduced risk of lung cancer [MIM:211980] in a population of Han Chinese subjects. Genetic vaiations are also associated with decreased risk of cancer of various other forms including esophageal, gastric, colorectal, cervical, and breast, acting in an allele dose-dependent manner.,PTM:Generation of the subunits requires association with the death-inducing signaling complex (DISC), whereas additional processing is likely due to the autocatalytic activity of the activated protease. GZMB and CASP10 can be involved in these processing events.,PTM:Phosphorylated upon DNA damage, probably by ATM or ATR.,similarity:Belongs to the peptidase C14A family.,similarity:Contains 2 DED (death effector) domains.,subunit:Heterotetramer that consists of two anti-parallel arranged heterodimers, each one formed by a 18 kDa (p18) and a 10 kDa (p10) subunit. Interacts with FADD, CFLAR and PEA15. Isoform 9 interacts at the endoplasmic reticulum with a complex containing BCAP31, BAP29, BCL2 and/or BCL2L1. Interacts with TNFAIP8L2.,tissue specificity:Isoforms 1, 5 and 7 are expressed in a wide variety of tissues. Highest expression in peripheral blood leukocytes, spleen, thymus, and liver. Barely detectable in brain, testis, and skeletal muscle.,
Research Area
p53;Apoptosis_Inhibition;Apoptosis_Mitochondrial;Apoptosis_Overview;Toll_Like;NOD-like receptor;RIG-I-like receptor;Alzheimer's disease;Huntington's disease;Pathways in cancer;Viral myocarditis;
