Summary
Performance
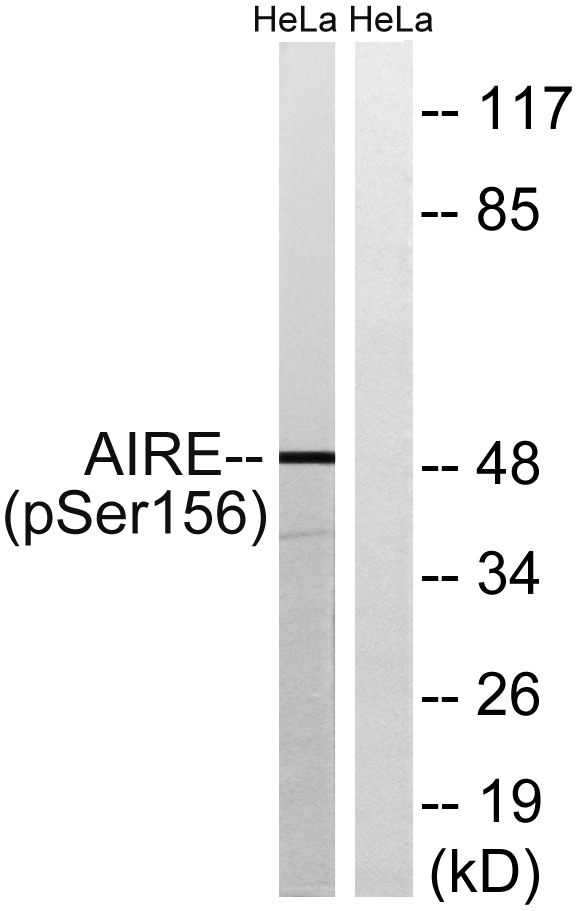
Immunogen
Application
Background
This gene encodes a transcriptional regulator that forms nuclear bodies and interacts with the transcriptional coactivator CREB binding protein. The encoded protein plays an important role in immunity by regulating the expression of autoantigens and negative selection of autoreactive T-cells in the thymus. Mutations in this gene cause the rare autosomal-recessive systemic autoimmune disease termed autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy (APECED). [provided by RefSeq, Jun 2012],alternative products:Additional isoforms seem to exist. Experimental confirmation may be lacking for some isoforms,disease:Defects in AIRE are a cause of autoimmune poly-endocrinopathy candidiasis ectodermal dystrophy (APECED) [MIM:240300]; also known as autoimmune polyglandular syndrome type I (APS-1). APECED is an autosomal recessive disease characterized by: (1) autoimmune polyendocrinopathies: hypoparathyroidism, adrenocortical failure, IDDM, gonadal failure, hypothyroidism, pernicious anemia, and hepatitis; (2) chronic mucocutaneous candidiasis; (3) ectodermal dystrophies: vitiligo, alopecia, keratopathy, dystrophy of dental enamel, nails and tympanic membranes. In addition, a high proportion of patients develop squamous cell carcinoma of the oral mucosa. The disease is reported worldwide but is exceptionally prevalent among the Finnish population (incidence 1:25000) and the Iranian jews (incidence 1:9000).,disease:Most of the mutations alter the nucleus-cytoplasm distribution of AIRE and disturb its association with nuclear dots and cytoplasmic filaments. Most of the mutations also decrease transactivation of the protein. The HSR domain is responsible for the homomultimerization activity of AIRE. All the missense mutations of the HSR and the SAND domains decrease this activity, but those in other domains do not. The AIRE protein is present in soluble high-molecular-weight complexes. Mutations in the HSR domain and deletion of PHD zinc fingers disturb the formation of these complexes.,domain:Disruption of the first PHD domain has been shown to lead to reduced transcriptional activity and to localization of the protein mainly in the cytoplasm in small granules. While the PHD zinc fingers are necessary for the transactivation capacity of the protein, other regions also modulate this function.,domain:The HSR domain is required for localization on tubular structures (N-terminal part) and for homodimerization.,domain:The L-X-X-L-L repeats may be implicated in binding to nuclear receptors.,function:Probable transcriptional regulator protein that binds to DNA as dimer and tetramer, but not as a monomer. Binds to G-doublets in an A/T-rich environment; the preferred motif is a tandem repeat of 5'-ATTGGTTA-3' combined with a 5'-TTATTA-3' box. May be involved in immune regulation.,online information:AIRE mutation db,PTM:Phosphorylated. Phosphorylation could trigger oligomerization.,similarity:Contains 1 HSR domain.,similarity:Contains 1 SAND domain.,similarity:Contains 2 PHD-type zinc fingers.,subcellular location:Associated with tubular structures and in discrete nuclear dots resembling ND10 nuclear bodies. May shuttle between nucleus and cytoplasm.,subunit:Homodimer and homotetramer. Interacts with CREBBP.,tissue specificity:Widely expressed. Expressed at higher level in thymus (medullary epithelial cells and monocyte-dendritic cells), pancreas, adrenal cortex, and testis. Expressed at lower level in the spleen, fetal liver and lymph nodes. Isoforms 2 and 3 seem to be less frequently expressed than isoform 1, if at all.,
Research Area
Ubiquitin mediated proteolysis;Primary immunodeficiency;