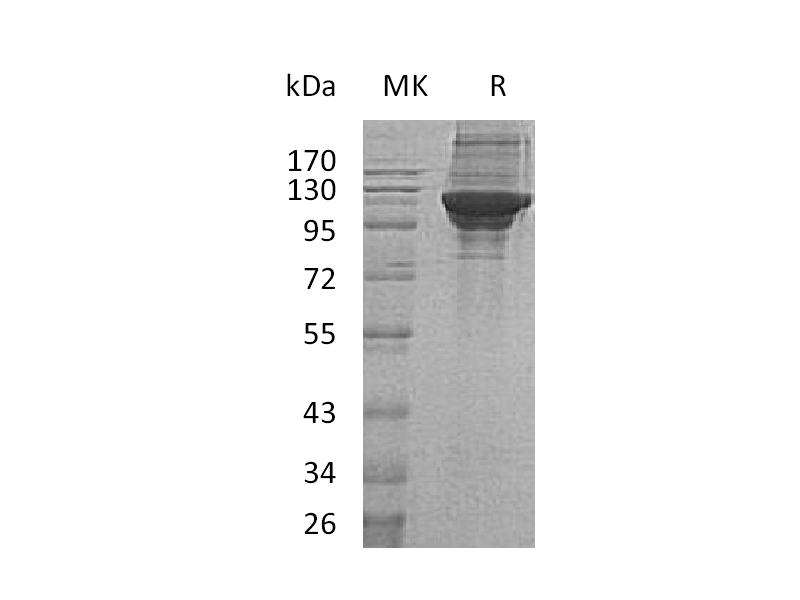
Alternative Names
Lysine--tRNA Ligase; Lysyl-tRNA Synthetase; LysRS; KARS; KIAA0070
Background
Lysine-tRNA ligase, also known as Lysyl-tRNA synthetase, LysRS, KARS and KIAA0070, belongs to the class-II aminoacyl-tRNA synthetase family. The N-terminal cytoplasmic domain (1-65) is a functional tRNA-binding domain, which is required for nuclear localization, is involved in the interaction with DARS, but has a repulsive role in the binding to EEF1A1. A central domain (208-259) is involved in homodimerization and is required for interaction with HIV-1 GAG and incorporation into virions. KARS catalyzes the specific attachment of an amino acid to its cognate tRNA in a two step reaction: the amino acid (AA) is first activated by ATP to form AA-AMP and then transferred to the acceptor end of the tRNA. Defects in KARS are the cause of Charcot-Marie-Tooth disease recessive intermediate type B (CMTRIB).
Note
For Research Use Only , Not for Diagnostic Use.