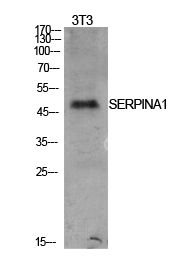
Summary
Performance
Immunogen
Application
Background
The protein encoded by this gene is secreted and is a serine protease inhibitor whose targets include elastase, plasmin, thrombin, trypsin, chymotrypsin, and plasminogen activator. Defects in this gene can cause emphysema or liver disease. Several transcript variants encoding the same protein have been found for this gene. [provided by RefSeq, Jul 2008],disease:Deficiency of the normal inhibitor in individuals homozygous for allele Z or M-Malton can result in the development of chronic emphysema or infantile liver cirrhosis.,disease:The major physiological function of AAT is the protection of the lower respiratory tract against proteolytic destruction by human leukocyte elastase (HLE). A hereditary deficiency of AAT, is associated with a 20-30 fold increased risk of developing chronic obstructive pulmonary disease.,disease:Variant Pittsburgh is the cause of bleeding diathesis.,domain:The reactive center loop (RCL) extends out from the body of the protein and directs binding to the target protease. The protease cleaves the serpin at the reactive site within the RCL, establishing a covalent linkage between the carboxyl group of the serpin reactive site and the serine hydroxyl of the protease. The resulting inactive serpin-protease complex is highly stable.,function:Inhibitor of serine proteases. Its primary target is elastase, but it also has a moderate affinity for plasmin and thrombin. Irreversibly inhibits trypsin, chymotrypsin and plasminogen activator. The aberrant form inhibits insulin-induced NO synthesis in platelets, decreases coagulation time and has proteolytic activity against insulin and plasmin.,function:Short peptide from AAT (SPAAT) is a reversible chymotrypsin inhibitor. It also inhibits elastase, but not trypsin.,miscellaneous:The aberrant form is found in the plasma of chronic smokers, and persists after smoking is ceased. It can still be found ten years after smoking has ceased.,online information:Alpha-1 antitrypsin entry,polymorphism:The sequence shown is that of the M1V allele which is the most common form of PI (44 to 49%). Other frequent alleles are: M1A 20 to 23%; M2 10 to 11%; M3 14 to 19%.,PTM:Proteolytic processing may yield the truncated form that ranges from Asp-30 to Lys-418.,PTM:Several isomers are observed, resulting from the combination of different N-linked glycan structures and mature N-terminus. N-linked glycan at Asn-107 is alternatively di-antennary, tri-antennary or tetra-antennary, whereas glycan at Asn-70 is di-antennary with trace amounts of tri-antennary, and glycan at Asn-271 is exclusively di-antennary. The structure of the antennas is Neu5Ac(alpha1-6)Gal(beta1-4)GlcNAc attached to the core structure Man(alpha1-6)[Man(alpha1-3)]Man(beta1-4)GlcNAc(beta1-4)GlcNAc. Some antennas are fucosylated, which forms a Lewis-X determinant.,similarity:Belongs to the serpin family.,tissue specificity:Plasma.,
Research Area
Complement and coagulation cascades;