Alternative Names
Hepatocyte growth factor receptor; HGF receptor; HGF/SF receptor; Proto-oncogene c-Met; Scatter factor receptor; SF receptor; Tyrosine-protein kinase Met; MET
Background
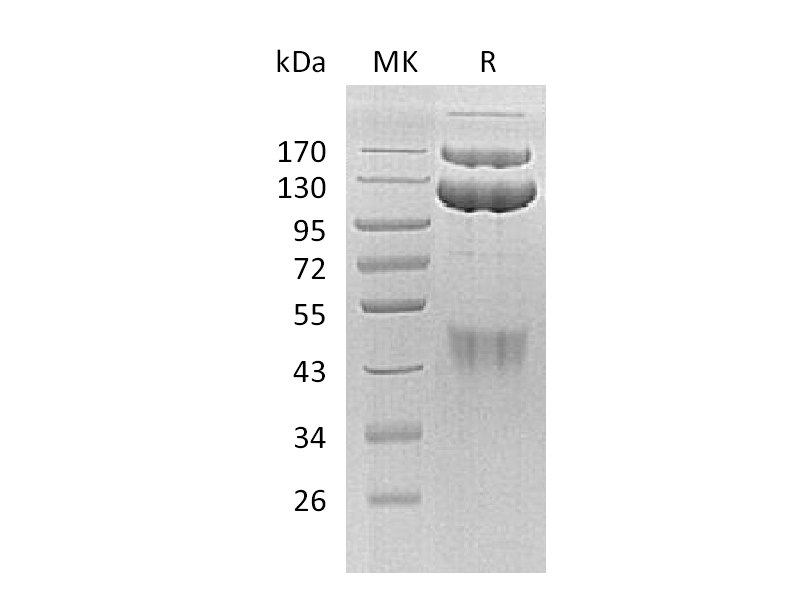
Hepatocyte growth factor receptor (HGF R) is a glycosylated receptor tyrosine kinase that plays a central role in epithelial morphogenesis and cancer development. HGF R is synthesized as a single chain precursor which undergoes cotranslational proteolytic cleavage. Mature HGF R is a disulfide-linked dimer composed of a 50 kDa extracellular α chain and a 145 kDa transmembrane β chain. Proteolysis and alternate splicing generate additional forms of human HGF R which either lack of the kinase domain, consist of secreted extracellular domains, or are deficient in proteolytic separation of the α and β chains. The sema domain, which is formed by both α and β chains of HGF R, mediates both ligand binding and receptor dimerization. HGF stimulation induces HGF R downregulation via internalization and proteasomedependent degradation. Paracrine induction of epithelial cell scattering and branching tubulogenesis results from the stimulation of HGF R on undifferentiated epithelium by HGF released from neighboring mesenchymal cells.
Note
For Research Use Only , Not for Diagnostic Use.