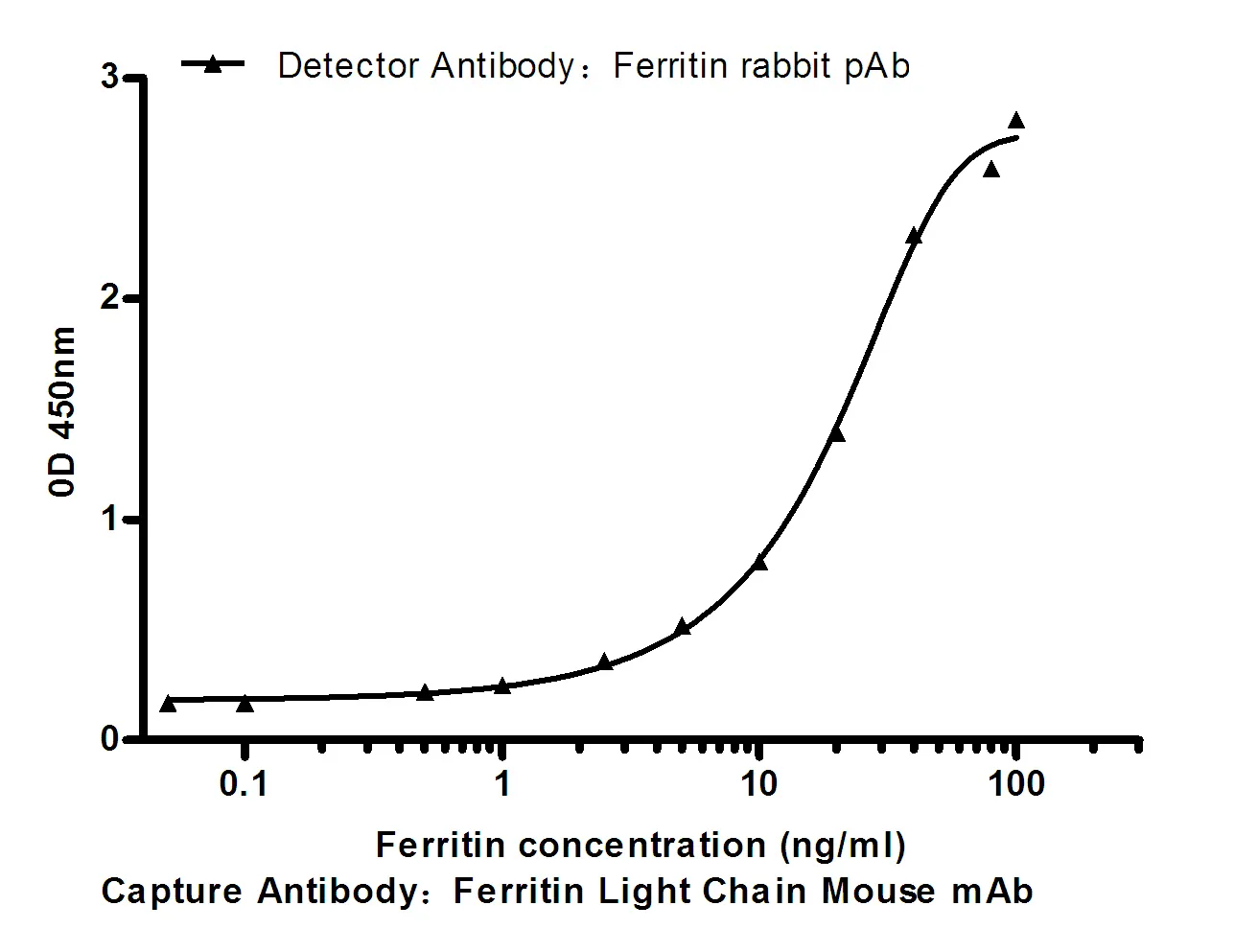
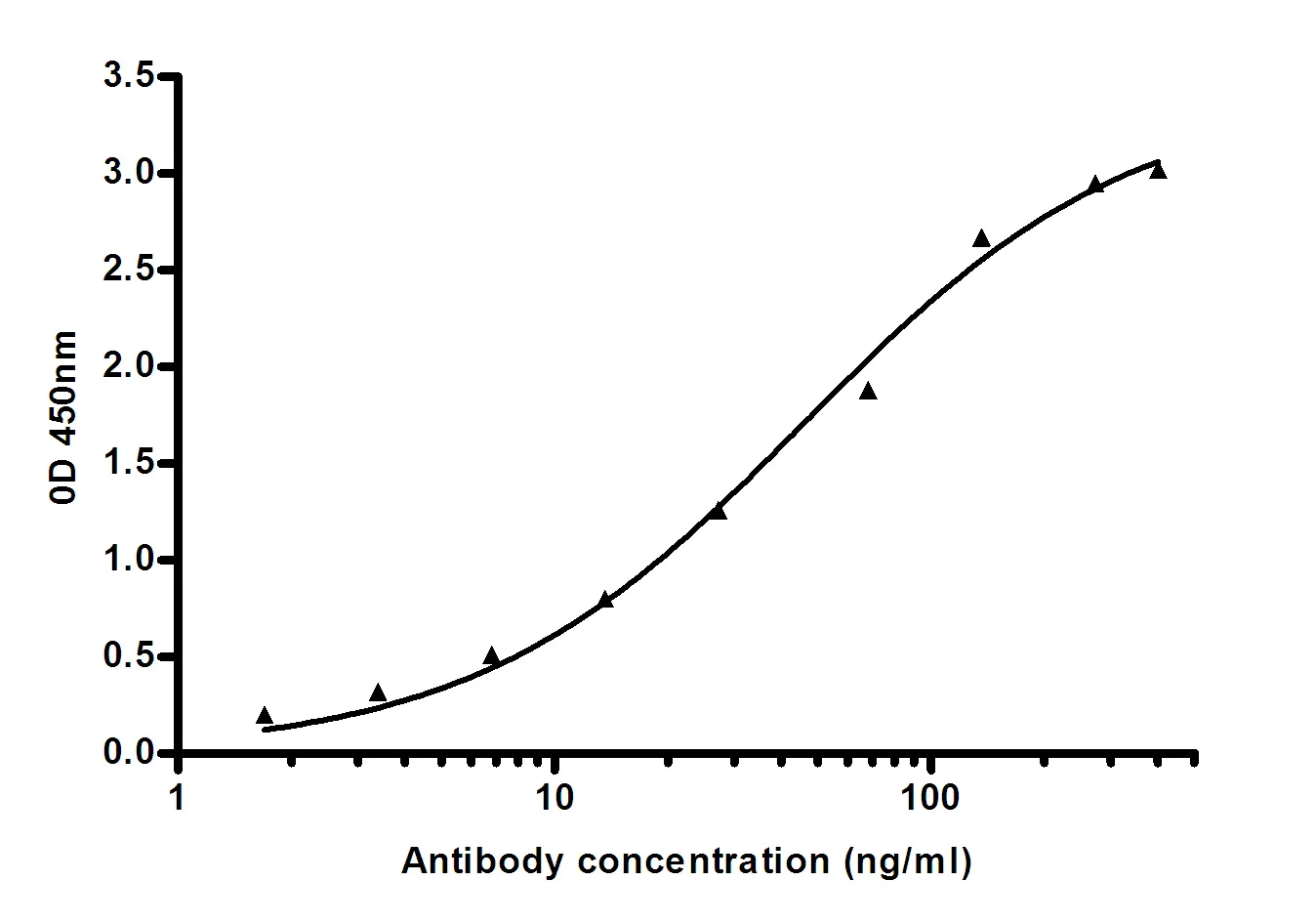
Summary
Performance
Immunogen
Application
Background
This gene encodes a multifunctional protein that resides in multiple locations in the nucleus and in the cytoplasm. It interacts with a wide variety of proteins, such as apoptosis antigen Fas, centromere protein C, and transcription factor erythroblastosis virus E26 oncogene homolog 1. In the nucleus, the encoded protein functions as a potent transcription repressor that binds to sumoylated transcription factors. Its repression can be relieved by the sequestration of this protein into promyelocytic leukemia nuclear bodies or nucleoli. This protein also associates with centromeres in G2 phase. In the cytoplasm, the encoded protein may function to regulate apoptosis. The subcellular localization and function of this protein are modulated by post-translational modifications, including sumoylation, phosphorylation and polyubiquitination. Alternative splicing results in multiple transcript varianfunction:Proposed to mediate activation of the JNK pathway and apoptosis via MAP3K5 in response to signaling from TNFRSF6 and TGFBR2. Interaction with HSPB1/HSP27 may prevent interaction with TNFRSF6 and MAP3K5 and block DAXX-mediated apoptosis. In contrast, in lymphoid cells JNC activation and TNFRSF6-mediated apoptosis may not involve DAXX. Seems to regulate transcription in PML/POD/ND10 nuclear bodies together with PML and may influence TNFRSF6-dependent apoptosis thereby. Down-regulates basal and activated transcription. Seems to act as a transcriptional co-repressor and inhibits PAX3 and ETS1 through direct protein-protein interaction. Modulates PAX5 activity. Its transcription repressor activity is modulated by recruiting it to subnuclear compartments like the nucleolus or PML/POD/ND10 nuclear bodies through interactions with MCSR1 and PML, respectively.,induction:Upon mitogenic stimulation by concanavalin A.,PTM:Phosphorylated upon DNA damage, probably by ATM or ATR. Phosphorylated by HIPK1 upon glucose deprivation.,PTM:Polyubiquitinated; which is promoted by CUL3 and SPOP and results in proteasomal degradation.,PTM:Sumoylated.,similarity:Belongs to the DAXX family.,subcellular location:Dispersed throughout the nucleoplasm, in PML/POD/ND10 nuclear bodies, and in nucleoli. Colocalizes with a subset of interphase centromeres, but is absent from mitotic centromeres. Detected in cytoplasmic punctate structures. Translocates from the nucleus to the cytoplasm upon glucose deprivation or oxidative stress.,subunit:Homomultimer. Binds to the TNFRSF6 death domain via its C-terminus and to PAX5. Binds to SLC2A4/GLUT4, MAP3K5, TGFBR2, phosphorylated dimeric HSPB1/HSP27, CENPC1, ETS1, sumoylated PML, UBE2I and MCRS1. Is part of a complex containing PAX5 and CREBBP. Interacts with HIPK2 and HIPK3 via its N-terminus. Interacts with HIPK1, which induces translocation from PML/POD/ND10 nuclear bodies to chromatin and enhances association with HDAC1 (By similarity). The non-phosphorylated form binds to PAX3, PAX7, DEK, HDAC1, HDAC2, HDAC3, acetylated histone H4 and histones H2A, H2B, H3 and H4. Interacts with SPOP. Part of a complex consisting of DAXX, CUL3 and SPOP. Interacts with CBP; the interaction is dependent the sumoylation of CBP and suppresses CBP transcriptional activity via recruitment of HDAC2 (By similarity). Interacts with HCMV tegument phosphoprotein pp71.,tissue specificity:Ubiquitous.,
Research Area
MAPK_ERK_Growth;MAPK_G_Protein;Amyotrophic lateral sclerosis (ALS);