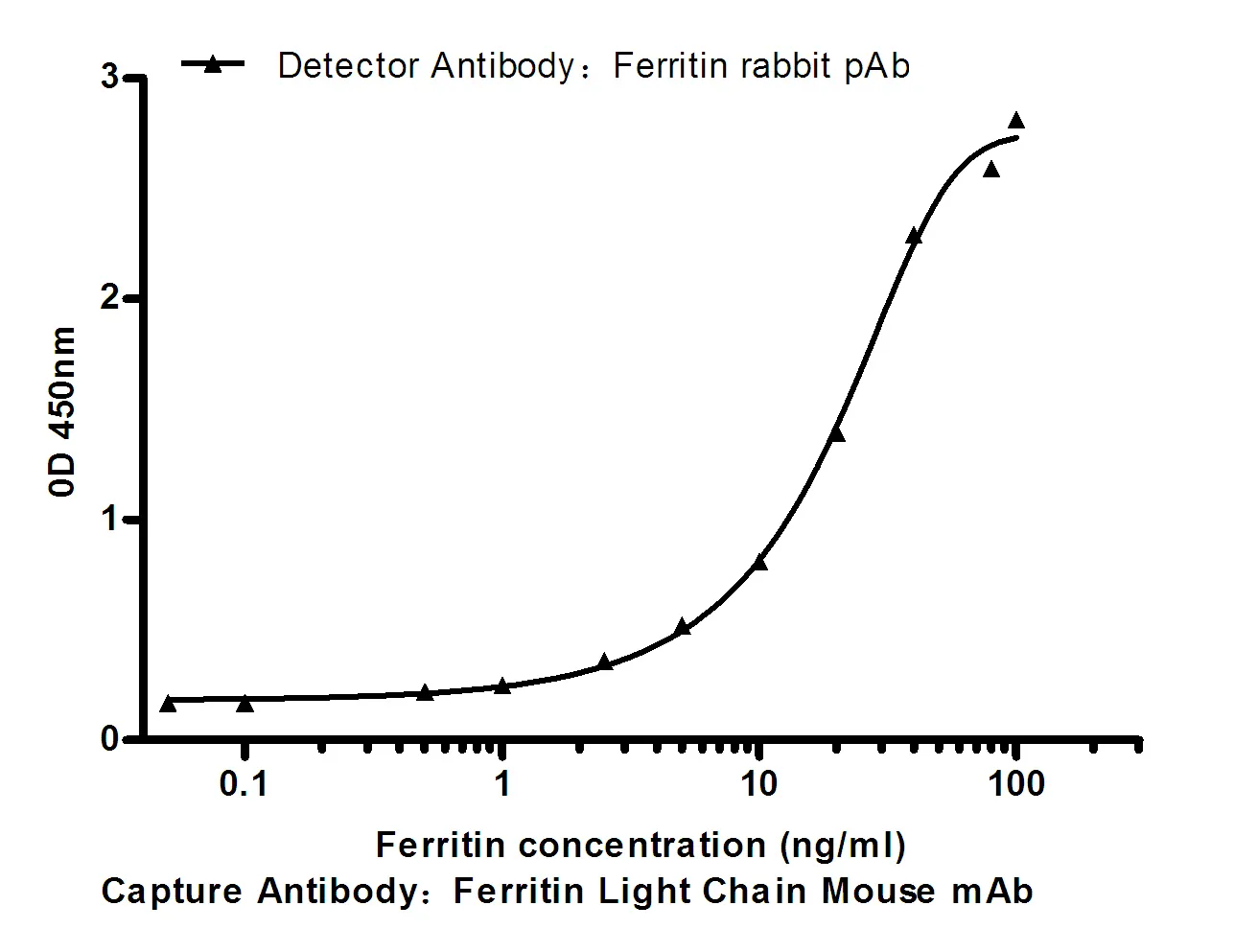
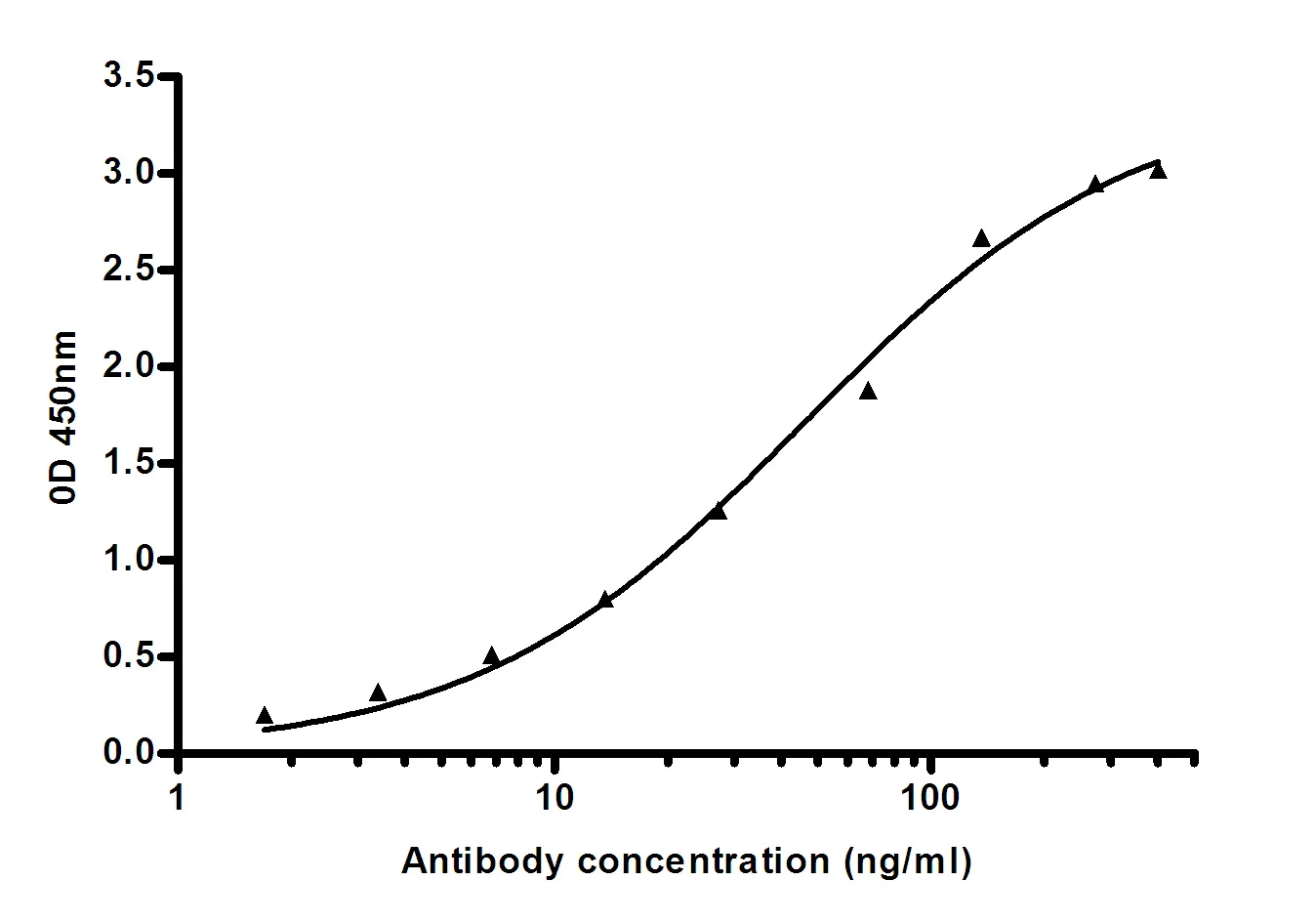
Summary
Performance
Immunogen
Application
Background
Aminopeptidase N is located in the small-intestinal and renal microvillar membrane, and also in other plasma membranes. In the small intestine aminopeptidase N plays a role in the final digestion of peptides generated from hydrolysis of proteins by gastric and pancreatic proteases. Its function in proximal tubular epithelial cells and other cell types is less clear. The large extracellular carboxyterminal domain contains a pentapeptide consensus sequence characteristic of members of the zinc-binding metalloproteinase superfamily. Sequence comparisons with known enzymes of this class showed that CD13 and aminopeptidase N are identical. The latter enzyme was thought to be involved in the metabolism of regulatory peptides by diverse cell types, including small intestinal and renal tubular epithelial cells, macrophages, granulocytes, and synaptic membranes from the CNS. Human aminopeptidase N iscatalytic activity:Release of an N-terminal amino acid, Xaa-|-Yaa- from a peptide, amide or arylamide. Xaa is preferably Ala, but may be most amino acids including Pro (slow action). When a terminal hydrophobic residue is followed by a prolyl residue, the two may be released as an intact Xaa-Pro dipeptide.,cofactor:Binds 1 zinc ion per subunit.,disease:Defects in ANPEP may be a cause of various types of leukemia or lymphoma.,domain:Amino acids 260-353 are essential to mediate susceptibility to infection with HCoV-229E (in porcine/human chimeric studies) and more specifically amino acids 288-295 (mutagenesis studies).,function:Broad specificity aminopeptidase. Plays a role in the final digestion of peptides generated from hydrolysis of proteins by gastric and pancreatic proteases. May play a critical role in the pathogenesis of cholesterol gallstone disease. May be involved in the metabolism of regulatory peptides of diverse cell types including small intestinal and tubular epithelial cells, macrophages, granulocytes and synaptic membranes from the CNS. Found to cleave antigen peptides bound to major histocompatibility complex class II molecules of presenting cells and to degrade neurotransmitters at synaptic junctions. Is also implicated as a regulator of IL-8 bioavailability in the endometrium, and therefore may contribute to the regulation of angiogenesis. Is used as a marker for acute myeloid leukemia and plays a role in tumor invasion. In case of human coronavirus 229E (HCoV-229E) infection, serves as receptor for HCoV-229E spike glycoprotein. Mediates as well human cytomegalovirus (HCMV) infection.,induction:Estradiol and IL-8 decrease enzymatic activity in vitro in endometrial stromal cells by 40% and 30%, respectively.,miscellaneous:Found to serve as a receptor for tumor-homing peptides, more specifically NGR peptides. It could serve thus as a target for delivering drugs into tumors. Concentration in human hepatic bile, varies from 17.3 to 57.6 micrograms/ml.,PTM:May undergo proteolysis and give rise to a soluble form.,PTM:N- and O-glycosylated.,PTM:Sulfated.,similarity:Belongs to the peptidase M1 family.,subcellular location:A soluble form has also been detected.,subunit:Homodimer. Interacts with the S1 domain of HCoV-229E spike protein.,tissue specificity:Expressed in epithelial cells of the kidney, intestine, and respiratory tract; granulocytes, monocytes, fibroblasts, endothelial cells, cerebral pericytes at the blood-brain barrier, synaptic membranes of cells in the CNS. Also expressed in endometrial stromal cells, but not in the endometrial glandular cells. Found in the vasculature of tissues that undergo angiogenesis and in malignant gliomas and lymph node metastases from multiple tumor types but not in blood vessels of normal tissues. A soluble form has been found in plasma. It is found to be elevated in plasma and effusions of cancer patients.,
Research Area
Glutathione metabolism;Renin-angiotensin system;Hematopoietic cell lineage;