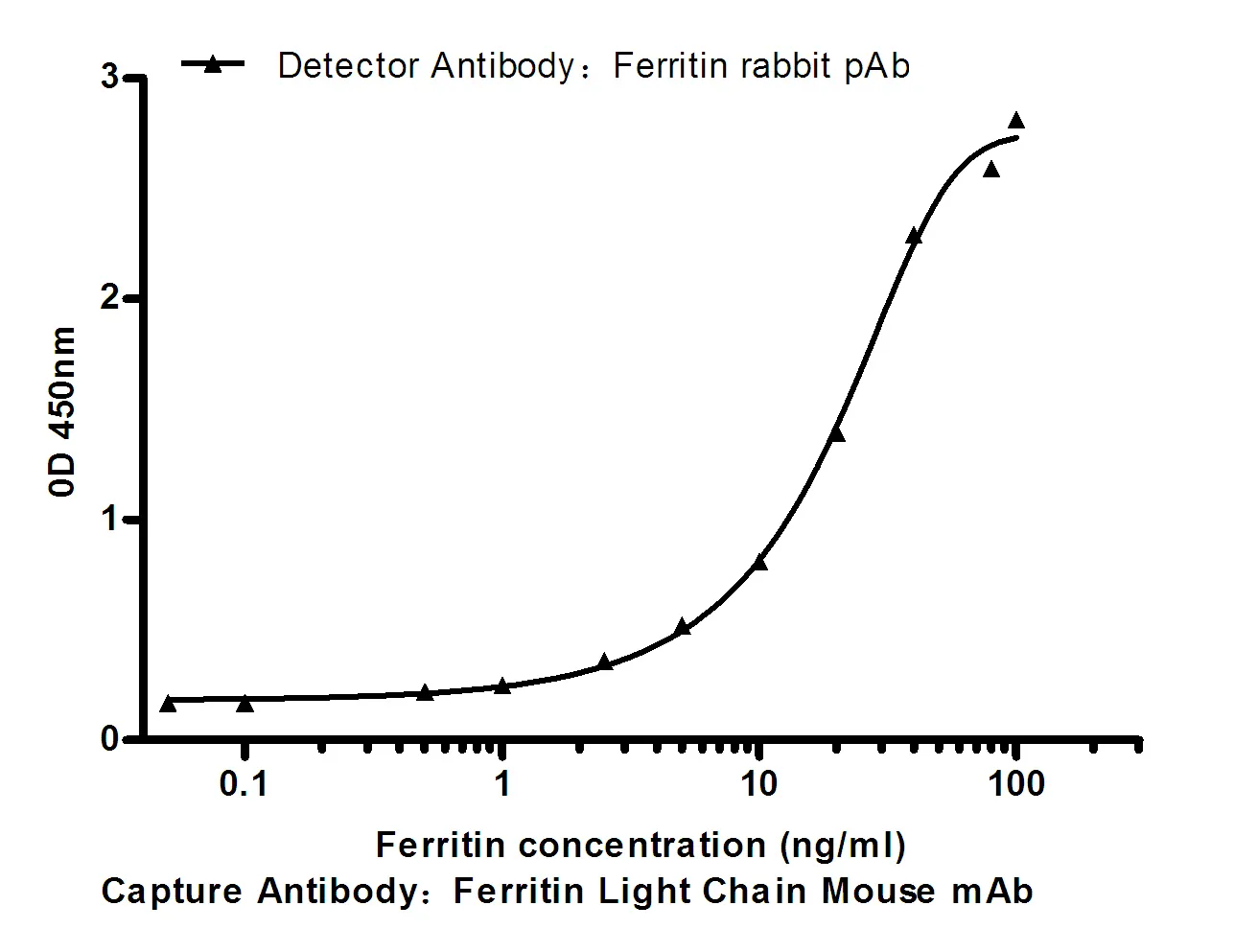
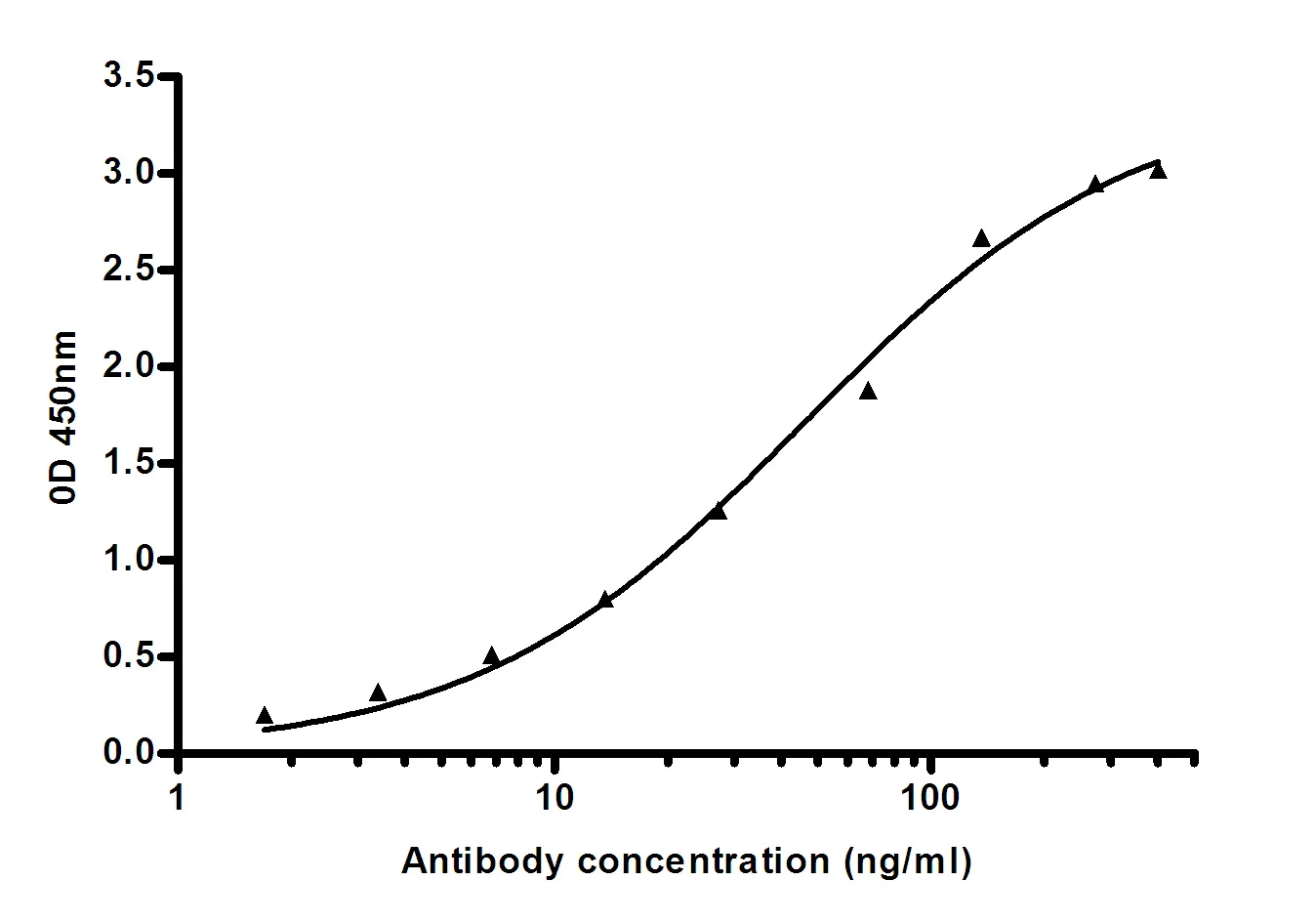
Summary
Performance
Immunogen
Application
Background
Proteins of the matrix metalloproteinase (MMP) family are involved in the breakdown of extracellular matrix in normal physiological processes, such as embryonic development, reproduction, and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. Most MMP's are secreted as inactive proproteins which are activated when cleaved by extracellular proteinases. However, the enzyme encoded by this gene is activated intracellularly by furin within the constitutive secretory pathway. Also in contrast to other MMP's, this enzyme cleaves alpha 1-proteinase inhibitor but weakly degrades structural proteins of the extracellular matrix. [provided by RefSeq, Jul 2008],cofactor:Binds 1 calcium ion per subunit.,cofactor:Binds 2 zinc ions per subunit.,domain:The conserved cysteine present in the cysteine-switch motif binds the catalytic zinc ion, thus inhibiting the enzyme. The dissociation of the cysteine from the zinc ion upon the activation-peptide release activates the enzyme.,function:May play an important role in the progression of epithelial malignancies.,PTM:The precursor is cleaved by a furin endopeptidase.,similarity:Belongs to the peptidase M10A family.,similarity:Contains 4 hemopexin-like domains.,tissue specificity:Specifically expressed in stromal cells of breast carcinomas.,
Research Area
Angiogenesis