Summary
Performance
Immunogen
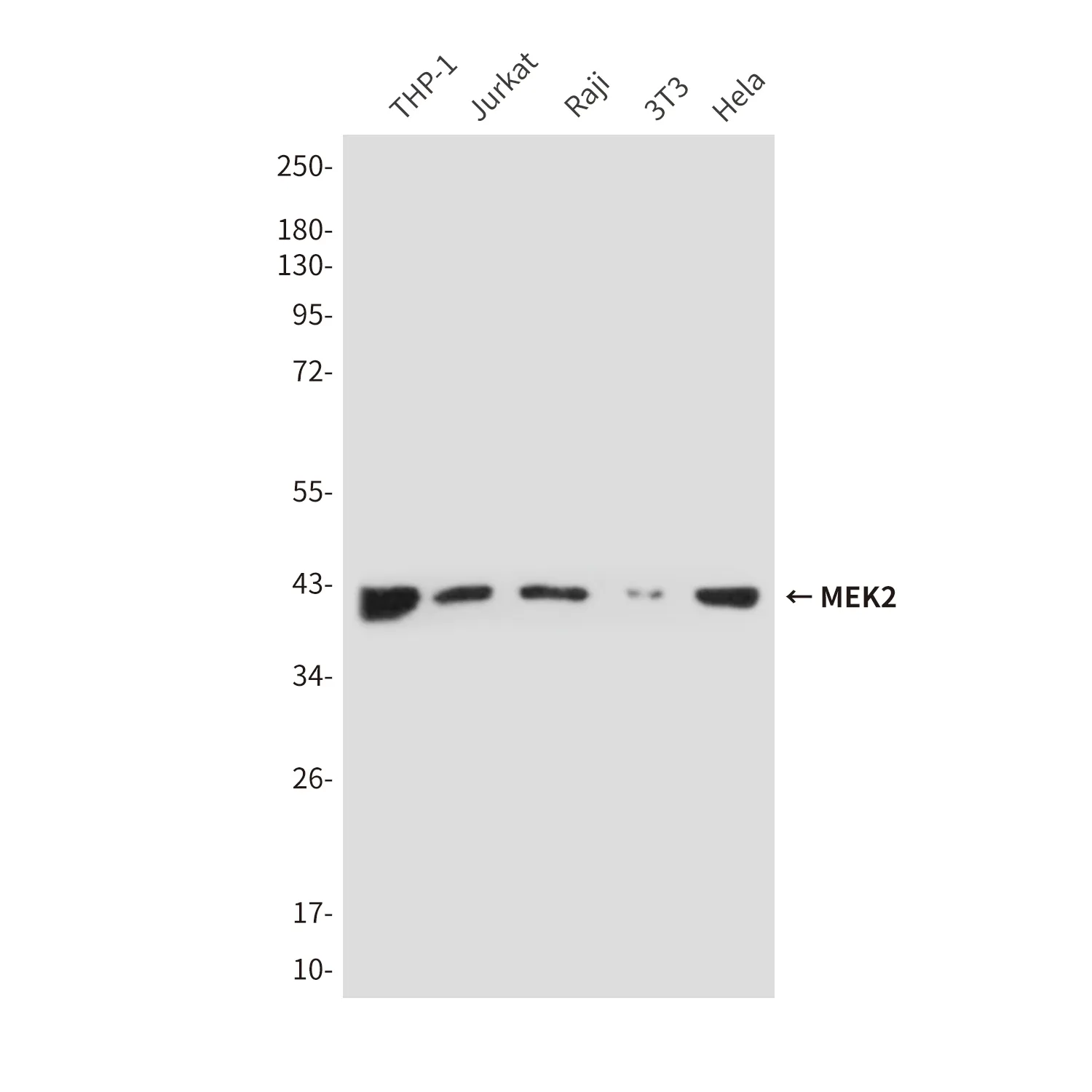
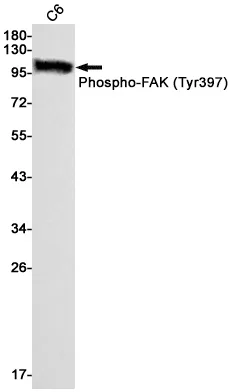
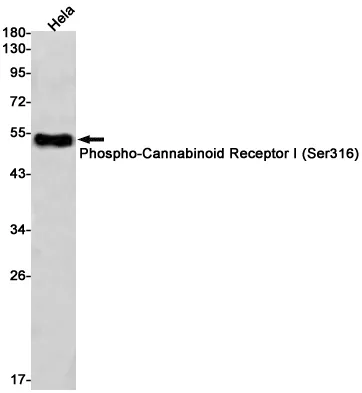
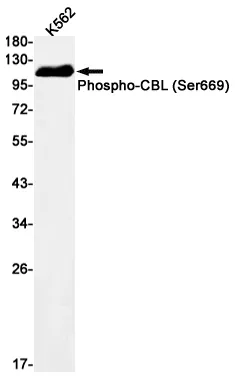
Application
Background
Conversion of pregnenolone and progesterone to their 17-alpha-hydroxylated products and subsequently to dehydroepiandrosterone (DHEA) and androstenedione. A cytochrome P450 monooxygenase involved in corticoid and androgen biosynthesis (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938). Catalyzes 17-alpha hydroxylation of C21 steroids, which is common for both pathways. A second oxidative step, required only for androgen synthesis, involves an acyl-carbon cleavage. The 17-alpha hydroxy intermediates, as part of adrenal glucocorticoids biosynthesis pathway, are precursors of cortisol (PubMed:9452426, PubMed:25301938) (Probable). Hydroxylates steroid hormones, pregnenolone and progesterone to form 17-alpha hydroxy metabolites, followed by the cleavage of the C17-C20 bond to form C19 steroids, dehydroepiandrosterone (DHEA) and androstenedione (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938). Has 16-alpha hydroxylase activity. Catalyzes 16-alpha hydroxylation of 17-alpha hydroxy pregnenolone, followed by the cleavage of the C17-C20 bond to form 16-alpha-hydroxy DHEA. Also 16-alpha hydroxylates androgens, relevant for estriol synthesis (PubMed:27339894, PubMed:25301938). Mechanistically, uses molecular oxygen inserting one oxygen atom into a substrate, and reducing the second into a water molecule, with two electrons provided by NADPH via cytochrome P450 reductase (CPR; NADPH-ferrihemoprotein reductase) (PubMed:9452426, PubMed:27339894, PubMed:22266943, PubMed:25301938).
Research Area