Alternative Names
Tryptophan synthetase; Tryptophan synthase
Background
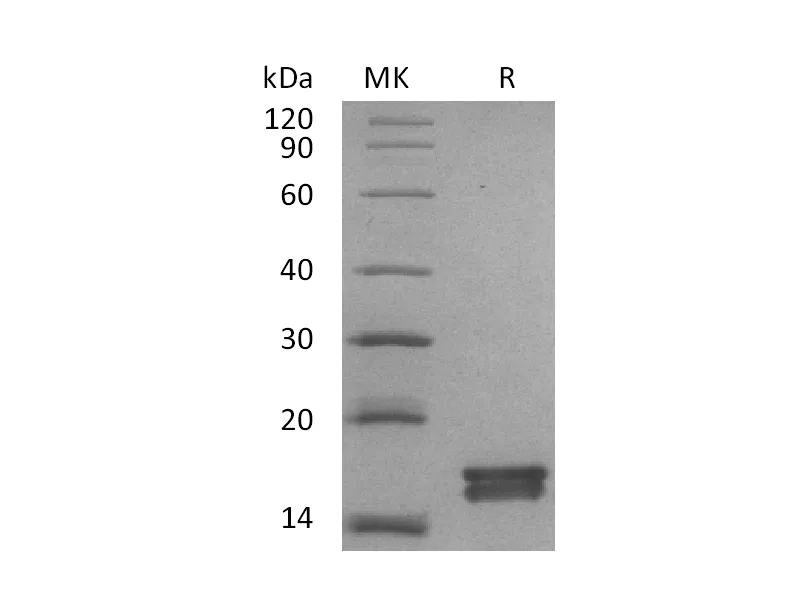
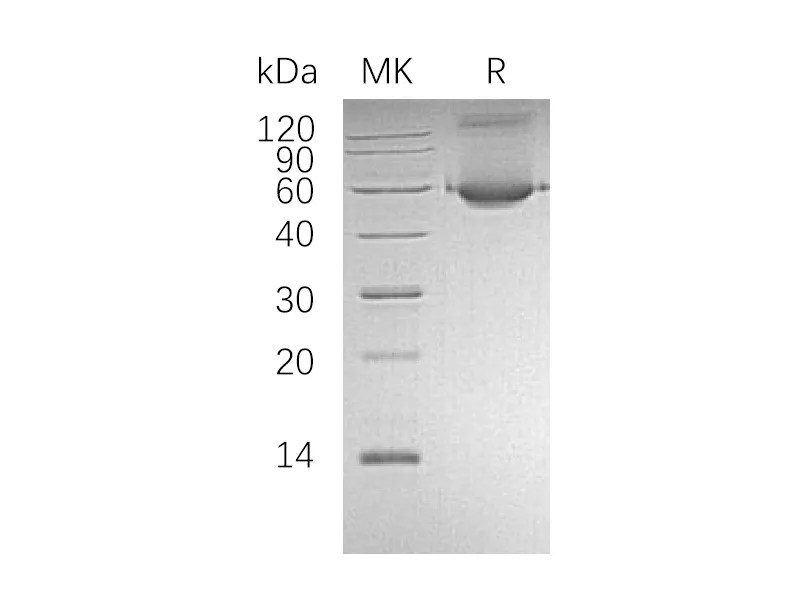
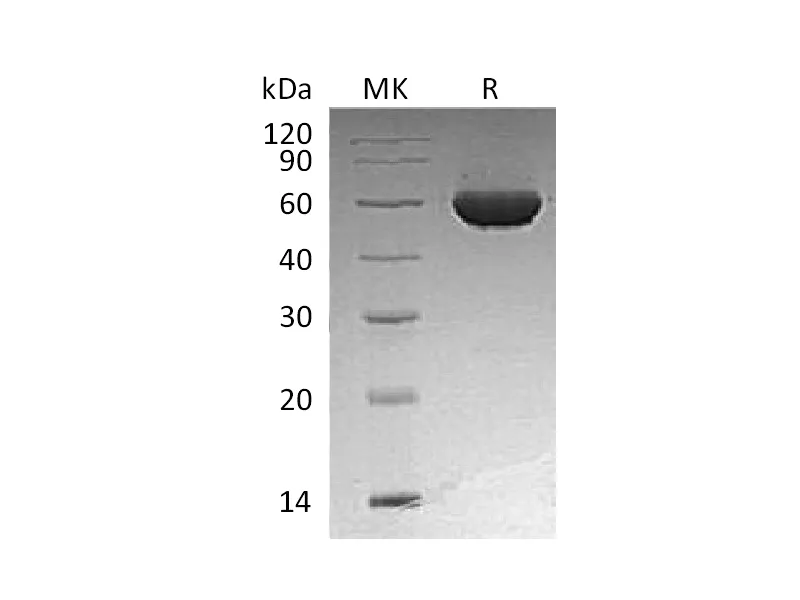
Tryptophan synthase is a multienzyme α2β2 complex composed of two protein subunit. Tryptophan synthase catalyzes the last two steps in the synthesis of L-tryptophan (L-Trp). The α-subunit catalyzes cleavage of 3-indole-d-glycerol 3′-phosphate (IGP) to give indole and D-glyceraldehyde 3′-phosphate (G3P). Indole is then transferred through a 25-Å hydrophobic tunnel to the β-subunit. The β2 subunit contains pyridoxal 5-phosphate and catalyzes several pyridoxal 5-phosphate-dependent reactions, including/3-elimination reactions 6 and a thiol-dependent transamination reaction. This enzyme is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae, but is absent from Animalia. As humans do not have tryptophan synthase, this enzyme has been explored as a potential drug target.
Note
For Research Use Only , Not for Diagnostic Use.