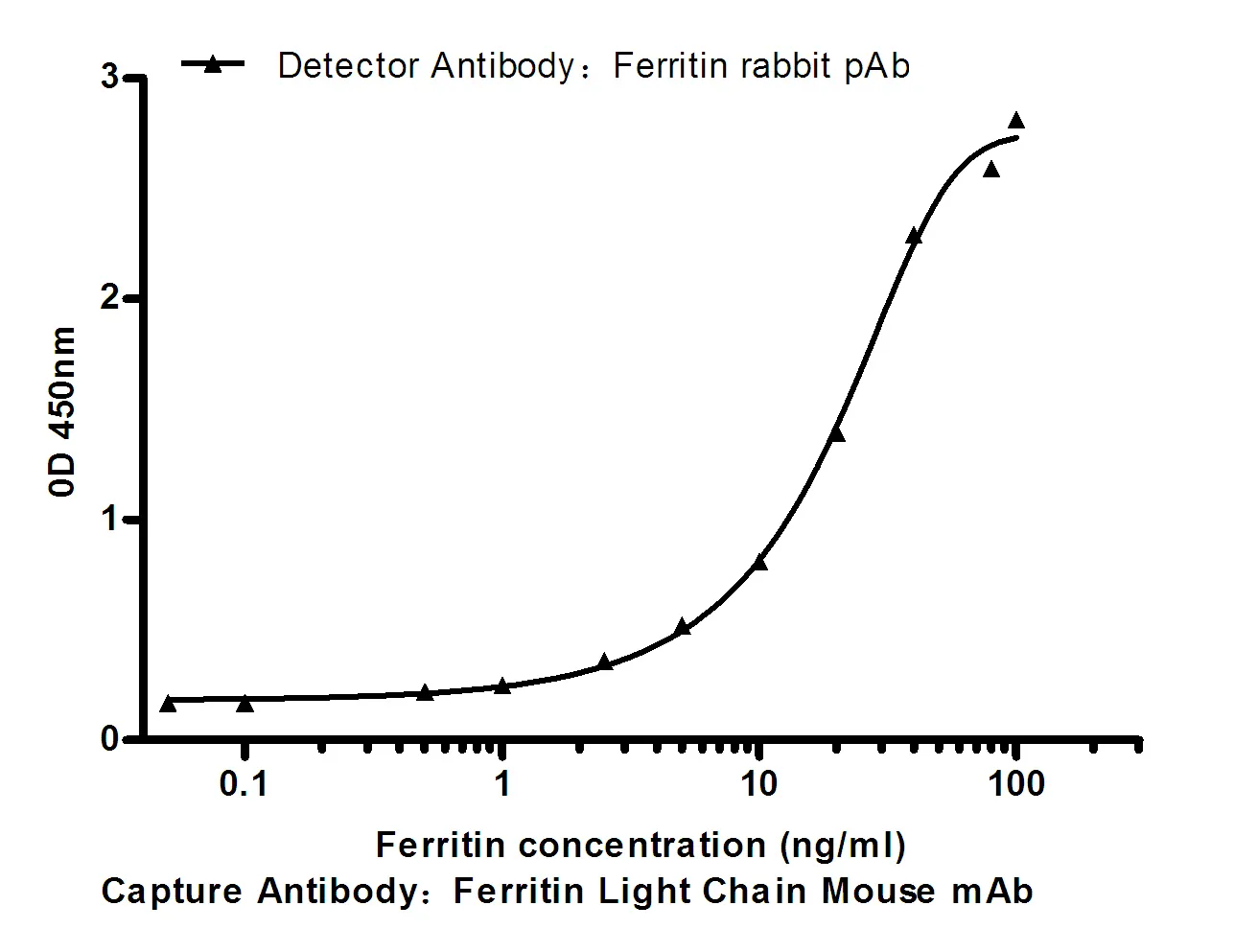
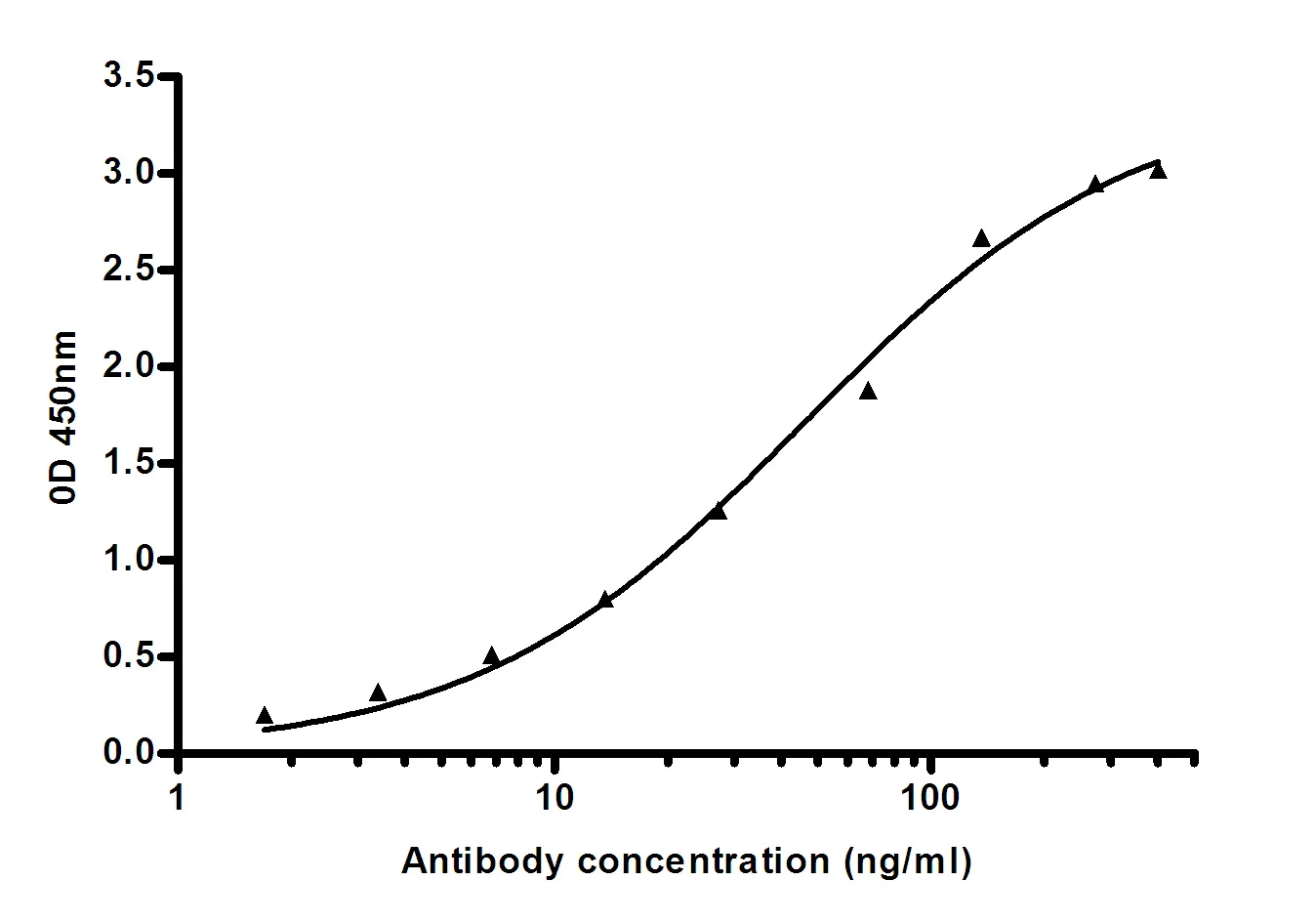
Summary
Performance
Immunogen
Application
Background
NEIL2 belongs to a class of DNA glycosylases homologous to the bacterial Fpg/Nei family. These glycosylases initiate the first step in base excision repair by cleaving bases damaged by reactive oxygen species and introducing a DNA strand break via the associated lyase reaction (Bandaru et al., 2002 [PubMed 12509226])[supplied by OMIM, Mar 2008],catalytic activity:Removes damaged bases from DNA, leaving an abasic site.,catalytic activity:The C-O-P bond 3' to the apurinic or apyrimidinic site in DNA is broken by a beta-elimination reaction, leaving a 3'-terminal unsaturated sugar and a product with a terminal 5'-phosphate.,domain:The zinc-finger domain is important for DNA binding.,enzyme regulation:Acetylation of Lys-50 leads to loss of DNA nicking activity. Acetylation of Lys-154 has no effect.,function:Involved in base excision repair of DNA damaged by oxidation or by mutagenic agents. Has DNA glycosylase activity towards 5-hydroxyuracil and other oxidized derivatives of cytosine with a preference for mismatched double stranded DNA (DNA bubbles). Has low or no DNA glycosylase activity towards thymine glycol, 2-hydroxyadenine, hypoxanthine and 8-oxoguanine. Has AP (apurinic/apyrimidinic) lyase activity and introduces nicks in the DNA strand. Cleaves the DNA backbone by beta-delta elimination to generate a single-strand break at the site of the removed base with both 3'- and 5'-phosphates.,similarity:Belongs to the FPG family.,similarity:Contains 1 FPG-type zinc finger.,subunit:Binds EP300.,tissue specificity:Detected in testis, skeletal muscle, heart, brain, placenta, lung, pancreas, kidney and liver.,
Research Area
Base excision repair;