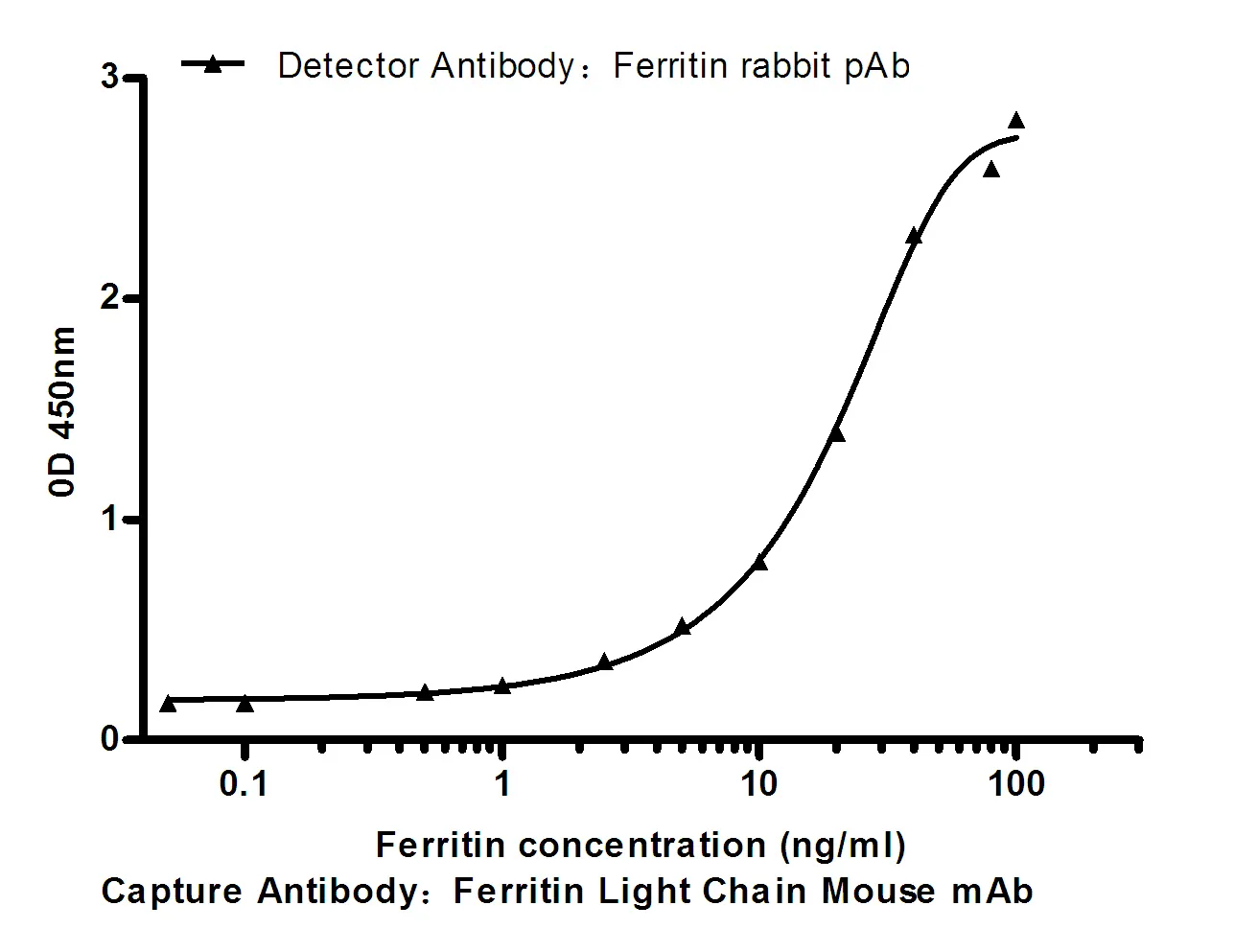
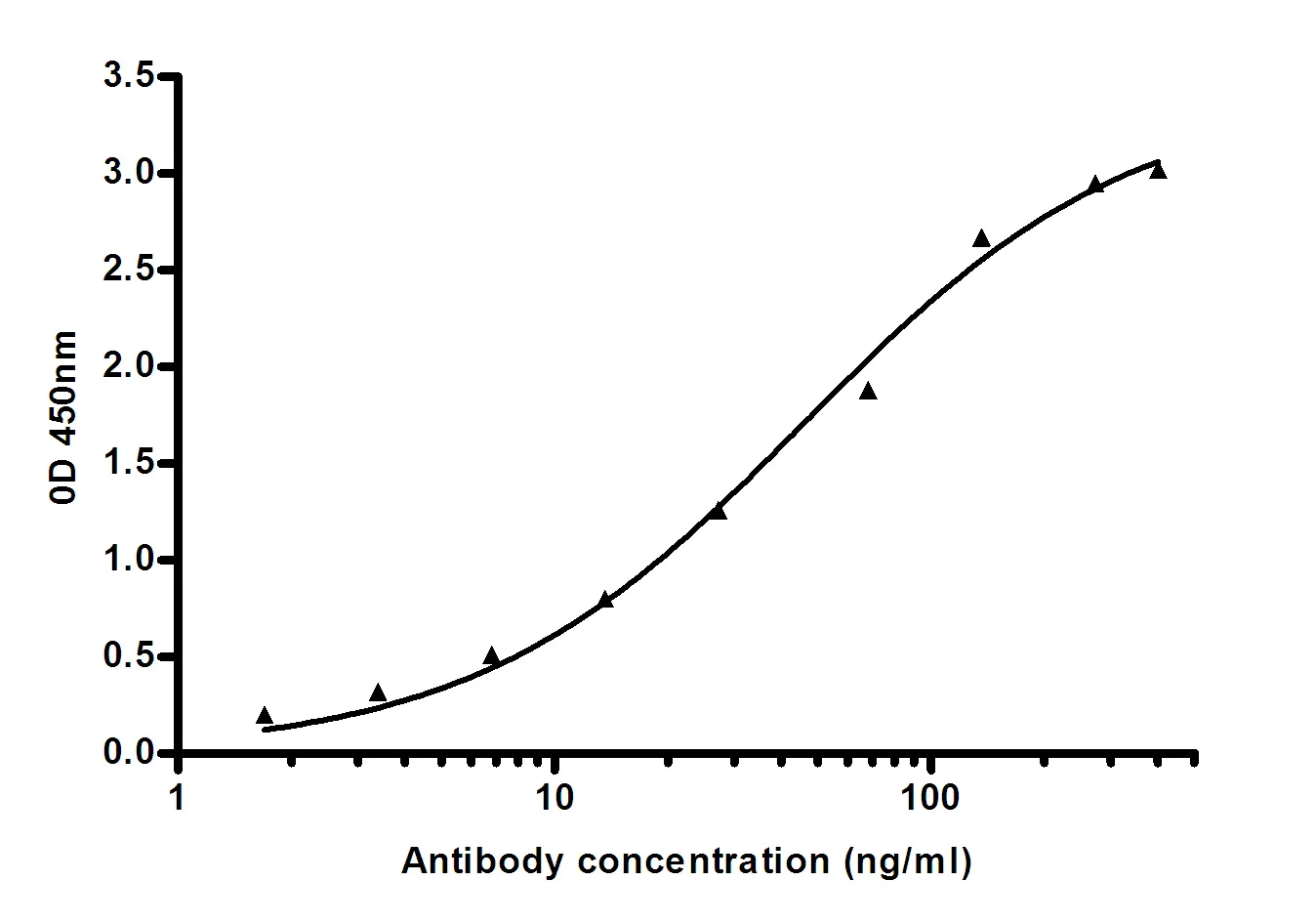
Summary
Performance
Immunogen
Application
Background
catalytic activity:Pyruvate + [dihydrolipoyllysine-residue acetyltransferase] lipoyllysine = [dihydrolipoyllysine-residue acetyltransferase] S-acetyldihydrolipoyllysine + CO(2).,cofactor:Thiamine pyrophosphate.,disease:Defects in PDHA1 are a cause of pyruvate decarboxylase E1 component deficiency (PDHE1 deficiency) [MIM:312170]. PDHE1 deficiency is the most common enzyme defect in patients with primary lactic acidosis. It is associated with variable clinical phenotypes ranging from neonatal death to prolonged survival complicated by developmental delay, seizures, ataxia, apnea, and in some cases to an X-linked form of Leigh syndrome (LS) (Leigh encephalomyelopathy).,disease:Defects in PDHA1 are the cause of X-linked Leigh syndrome (LS) [MIM:308930]. LS is an early-onset progressive neurodegenerative disorder with a characteristic neuropathology consisting of focal, bilateral lesions in one or more areas of the central nervous system, including the brainstem, thalamus, basal ganglia, cerebellum, and spinal cord. The lesions are areas of demyelination, gliosis, necrosis, spongiosis, or capillary proliferation. Clinical symptoms depend on which areas of the central nervous system are involved. The most common underlying cause is a defect in oxidative phosphorylation. LS may be a feature of a deficiency of any of the mitochondrial respiratory chain complexes.,enzyme regulation:E1 activity is regulated by phosphorylation (inactivation) and dephosphorylation (activation) of the alpha subunit.,function:The pyruvate dehydrogenase complex catalyzes the overall conversion of pyruvate to acetyl-CoA and CO(2). It contains multiple copies of three enzymatic components: pyruvate dehydrogenase (E1), dihydrolipoamide acetyltransferase (E2) and lipoamide dehydrogenase (E3).,subunit:Tetramer of 2 alpha and 2 beta subunits.,tissue specificity:Ubiquitous.,
Research Area