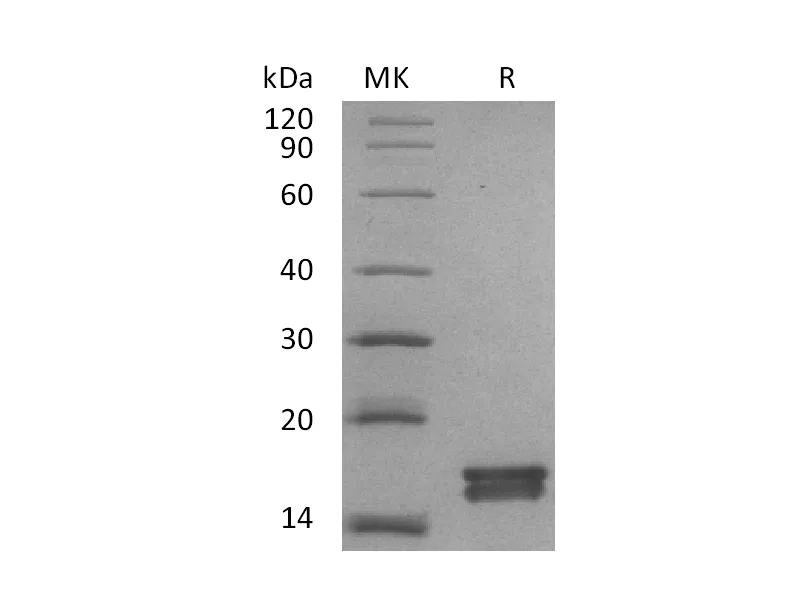
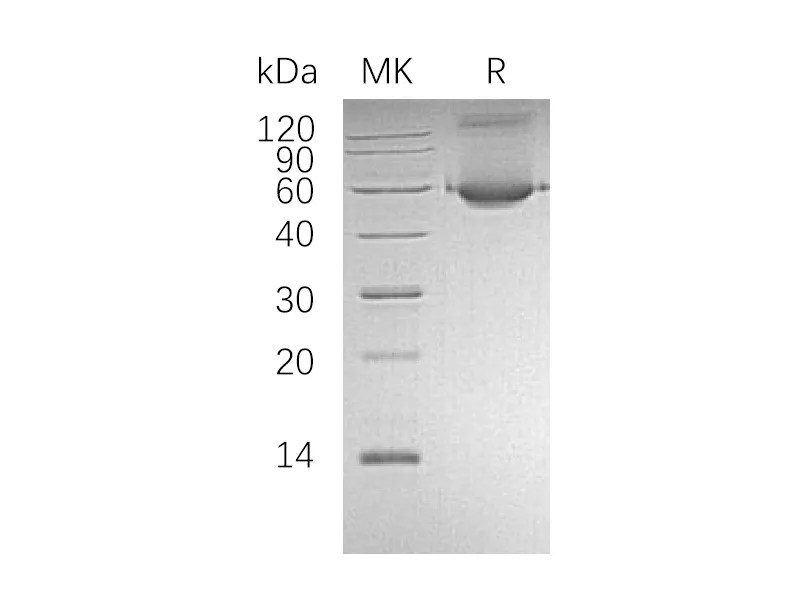
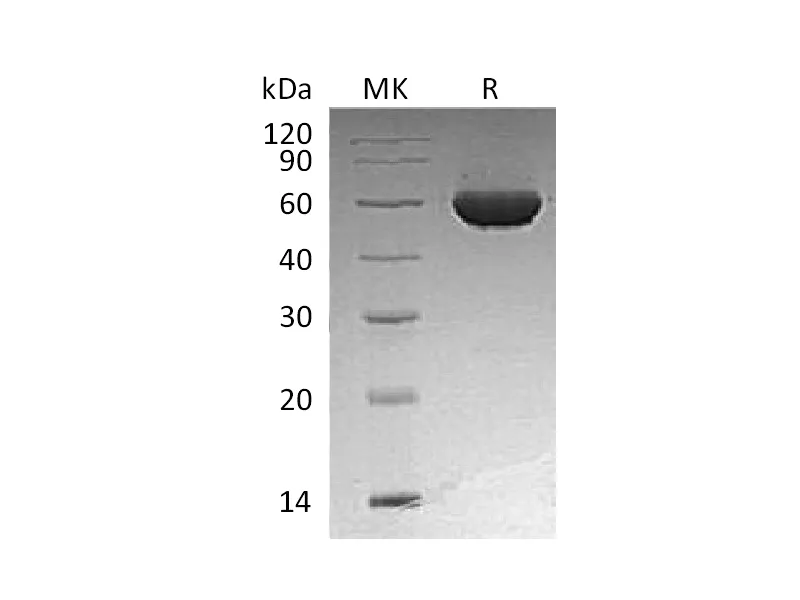
Alternative Names
B3GAT1; beta-1,3-glucuronyltransferase 1 (glucuronosyltransferase P); CD57; GlcAT-P; HNK1; NK1; NK-1
Background
B3GAT1 is the key enzyme during the biosynthesis of the carbohydrate epitope HNK-1, which is present on a number of cell adhesion molecules important in neurodevelopment. It adds a glucuronic residue to the terminal lactosamine residue (Gal beta 14GlcNAc) of a glycoprotein or glycolipid, which can be further sulfated to become the HNK1 epitope, a unique trisaccharide structure, HSO3-3GlcA beta 1-3Gal beta 1-4GlcNAc. The enzyme activity was found to be enhanced in the presence of sphingomyelin and phosphatidylinositol. The HNK1 carbohydrate epitope is characteristically expressed on a series of cell adhesion molecules in addition to some glycolipids in the extracellular matrix and on the cell surface in the nervous system, where it is involved in cell-cell and cell-substratum interaction and recognition during the development of the nervous system. Like most known glycosyltransferases, B3GAT1 is a type II Golgi-resident transmembrane protein with a short N-terminal cytoplasmic domain and a single pass transmembrane domain followed by an enzymatic domain in the lumen of Golgi apparatus. The enzyme activity was assayed using a phosphatase-coupled method.
Note
For Research Use Only , Not for Diagnostic Use.