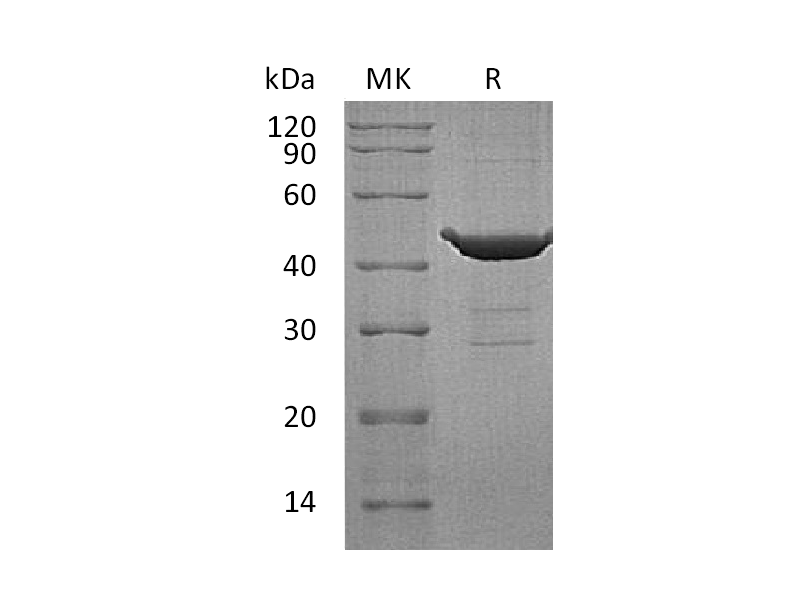
| Name | Recombinant Human RheB (N-GST) |
| Purity | Greater than 95% as determined by reducing SDS-PAGE |
| Endotoxin level | <1 EU/µg as determined by LAL test. |
| Construction | Recombinant Human Ras Homolog Enriched In Brain is produced by our E.coli expression system and the target gene encoding Met1-Met184 is expressed with a GST tag at the N-terminus. |
| Accession # | Q15382 |
| Host | E.coli |
| Species | Human |
| Predicted Molecular Mass | 20.4 KDa |
| Buffer | Lyophilized from a 0.2 μm filtered solution of 20mM PB, 8% Trehalose, 4% Mannitol, 50mM NaCl, 10mM GSH, 0.05% Tween 80, pH6.5. |
| Form | Lyophilized |
| Shipping | The product is shipped at ambient temperature.Upon receipt, store it immediately at the temperature listed below. |
| Stability&Storage | Store at ≤-70°C, stable for 6 months after receipt.Store at ≤-70°C, stable for 3 months under sterile conditions after opening. Please minimize freeze-thaw cycles. |
| Reconstitution | Always centrifuge tubes before opening.Do not mix by vortex or pipetting.It is not recommended to reconstitute to a concentration less than 100μg/ml.Dissolve the lyophilized protein in distilled water.Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
Alternative Names
GTP-Binding Protein Rheb; Ras Homolog Enriched in Brain; RHEB; RHEB2
Background
GTP-Binding Protein Rheb (RHEB) is a member of the small GTPase superfamily and encodes a lipid-anchored, cell membrane protein with five repeats of the RAS-related GTP-binding region. Highest levels of RHEB can be found in the skeletal and cardiac muscle, and it is vital in the regulation of growth and cell cycle progression due to its role in the Insulin/TOR/S6K signaling pathway. RHEB stimulates the phosphorylation of S6K1 and EIF4EBP1 through activation of mTORC1 signaling, and it activates the protein kinase activity of mTORC1. RHEB has GTPase activity and shuttles between a GDP-bound form and a GTP-bound form, farnesylation of the protein is required for this activity.
Note
For Research Use Only , Not for Diagnostic Use.