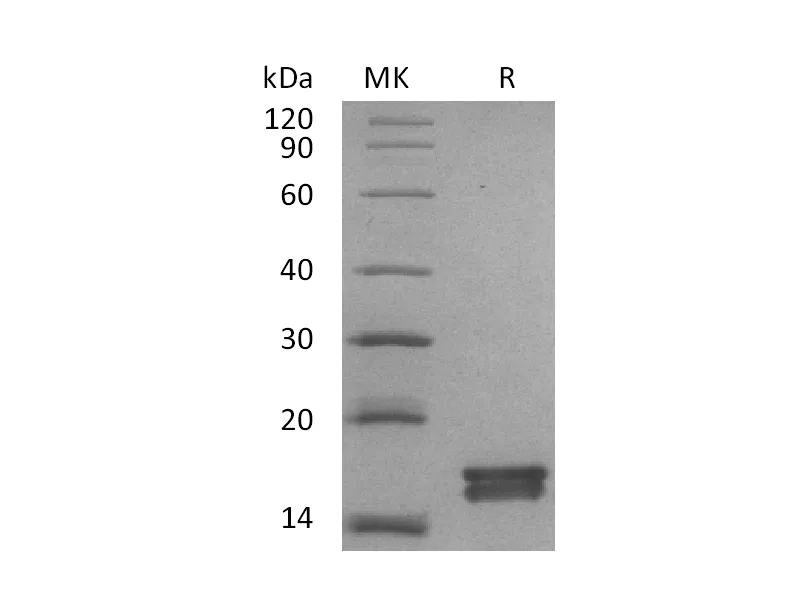
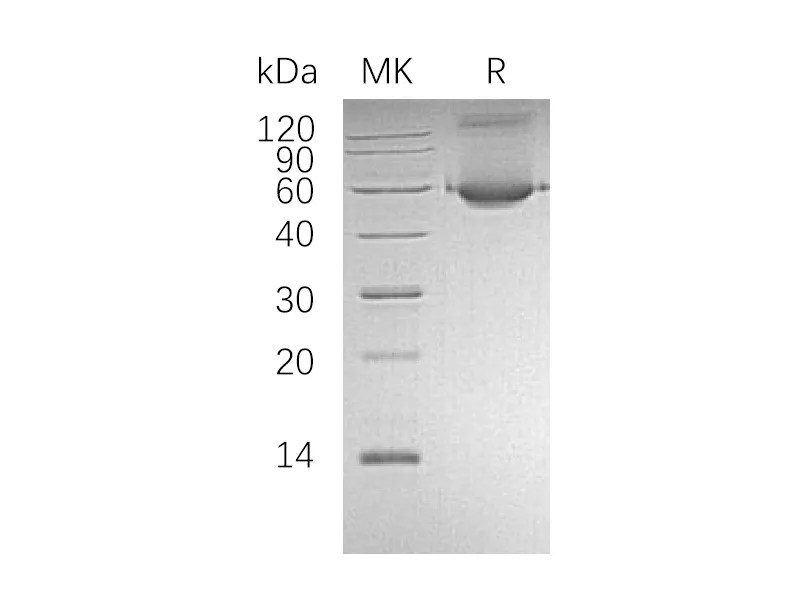
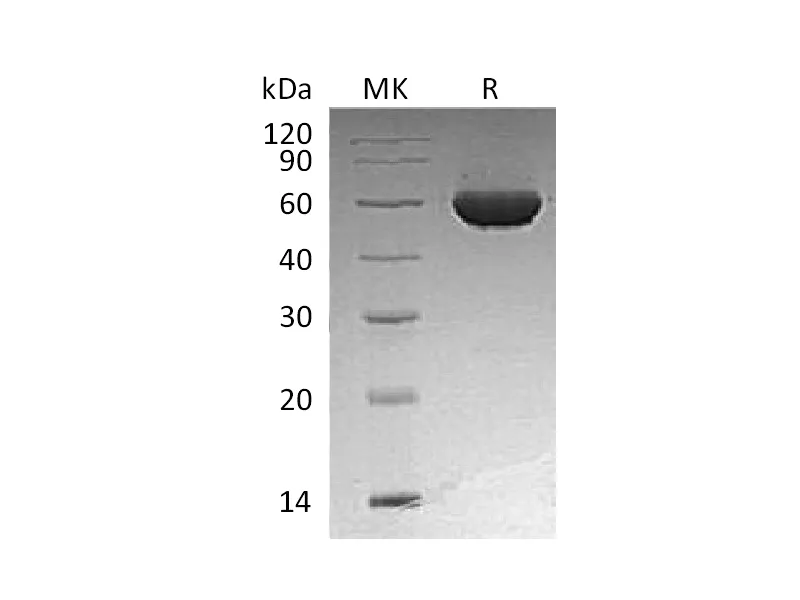
Alternative Names
Myelin-Associated Glycoprotein; MAG; Siglec-4a
Background
Myelin-Associated Glycoprotein (MAG, Siglec-4a), is a type I transmembrane glycoprotein belonging to the Siglec family. It is composed of an extracellular segment containing five Ig-like domains, a single transmembrane segment, and a cytoplasmic domain. Mouse MAG shares 95% and 99% aa sequence identity with human and rat MAG, respectively. MAG functions as an adhesion molecule during neural development. It preferentially binds to alpha -2,3-linked sialic acid terminal structures found on cell surface molecules. MAG is selectively expressed by myelinating oligodendrocytes and Schwann cells and plays an important role in axon-myelin stability. MAG is also reported to regulate the axon cytoskeleton and support the distribution of axon molecules at the nodes of Ranvier. In addition, it has been identified as a major inhibitor of neurite outgrowth.
Note
For Research Use Only , Not for Diagnostic Use.