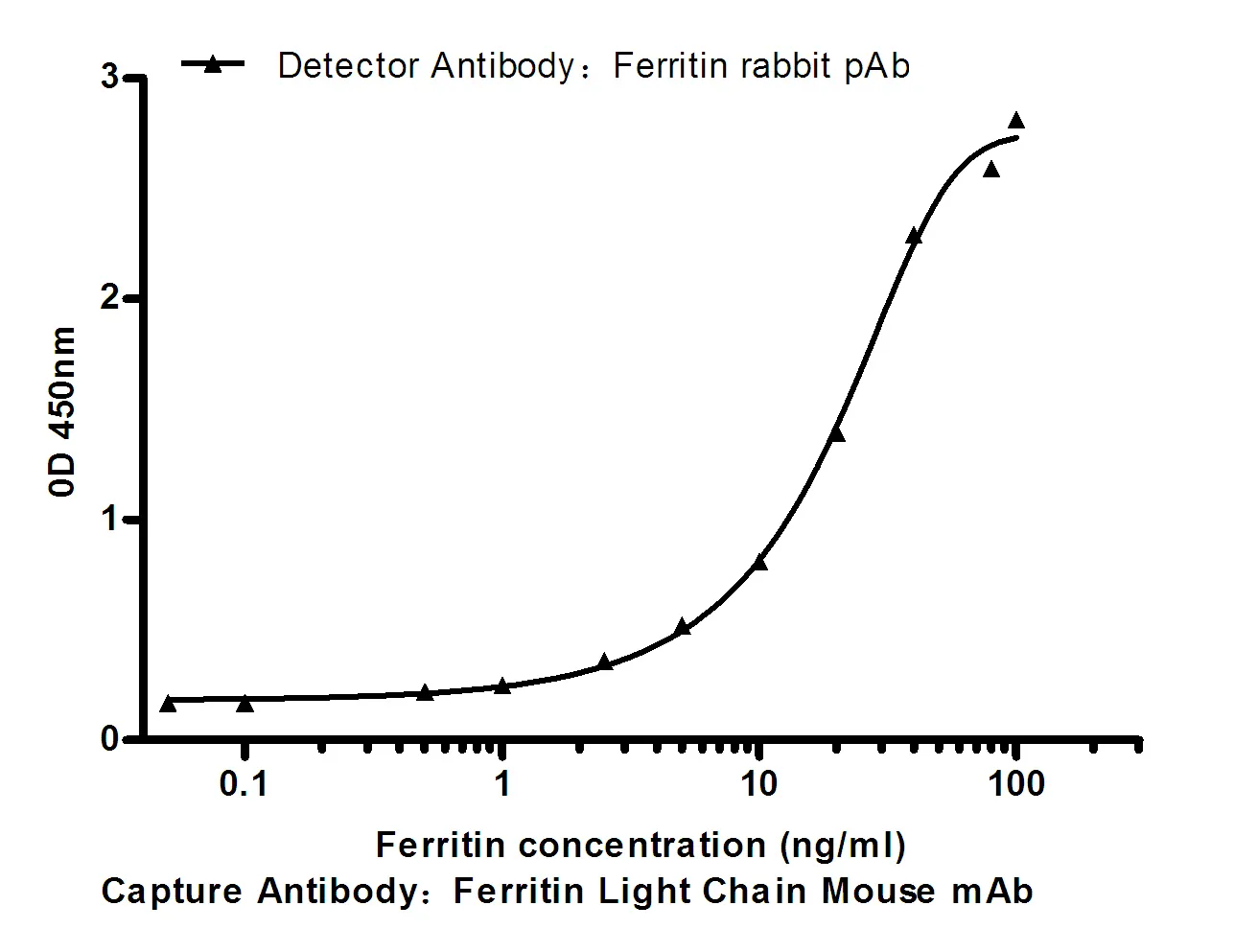
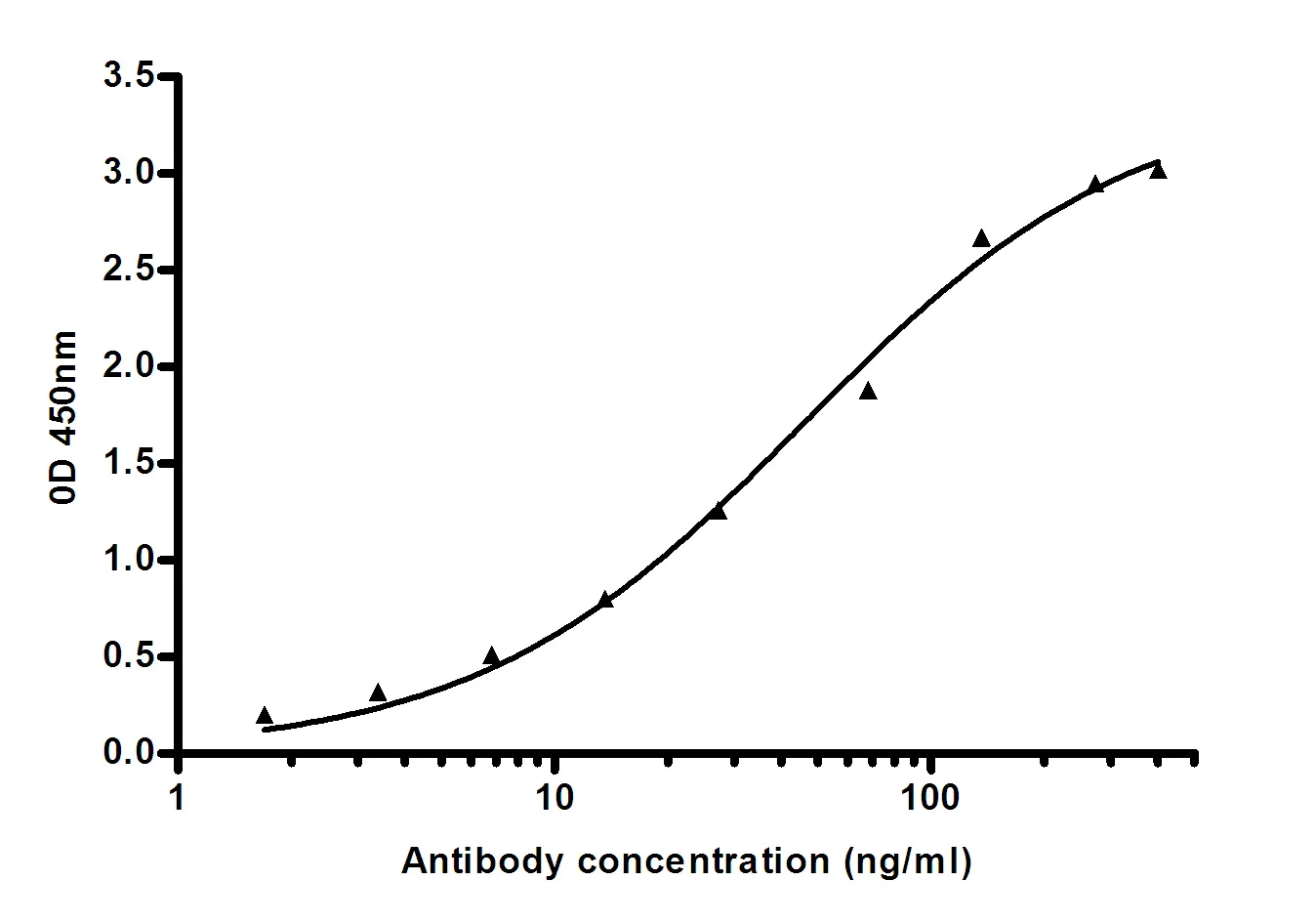
Summary
Performance
Immunogen
Application
Background
Cbl proto-oncogene(CBL) Homo sapiens This gene is a proto-oncogene that encodes a RING finger E3 ubiquitin ligase. The encoded protein is one of the enzymes required for targeting substrates for degradation by the proteasome. This protein mediates the transfer of ubiquitin from ubiquitin conjugating enzymes (E2) to specific substrates. This protein also contains an N-terminal phosphotyrosine binding domain that allows it to interact with numerous tyrosine-phosphorylated substrates and target them for proteasome degradation. As such it functions as a negative regulator of many signal transduction pathways. This gene has been found to be mutated or translocated in many cancers including acute myeloid leukaemia, and expansion of CGG repeats in the 5' UTR has been associated with Jacobsen syndrome. Mutations in this gene are also the cause of Noonan syndrome-like disorder. [provided by RefSeq, Jul 2016],disease:Can be converted to an oncogenic protein by deletions or mutations that disturb its ability to down-regulate RTKs.,domain:The N-terminus is composed of the phosphotyrosine binding (PTB) domain, a short linker region and the RING-type zinc finger. The PTB domain, which is also called TKB (tyrosine kinase binding) domain, is composed of three different subdomains: a four-helix bundle (4H), a calcium-binding EF hand and a divergent SH2 domain.,domain:The RING-type zinc finger domain mediates binding to an E2 ubiquitin-conjugating enzyme.,function:Participates in signal transduction in hematopoietic cells. Adapter protein that functions as a negative regulator of many signaling pathways that start from receptors at the cell surface. Acts as an E3 ubiquitin-protein ligase, which accepts ubiquitin from specific E2 ubiquitin-conjugating enzymes, and then transfers it to substrates promoting their degradation by the proteasome. Recognizes activated receptor tyrosine kinases, including PDGFA, EGF and CSF1, and terminates signaling.,miscellaneous:This protein has one functional calcium-binding site.,pathway:Protein modification; protein ubiquitination.,PTM:Phosphorylated on tyrosine residues by EGFR, SYK, FYN and ZAP70 (By similarity). Phosphorylated on tyrosine residues by INSR.,similarity:Contains 1 CBL N-terminal domain.,similarity:Contains 1 RING-type zinc finger.,similarity:Contains 1 SH2 domain.,similarity:Contains 1 UBA domain.,similarity:Contains 2 EF-hand-like domains.,subunit:Associates with NCK via its SH3 domain. The phosphorylated C-terminus interacts with CD2AP via its second SH3 domain. Binds to UBE2L3. Interacts with adapters SLA, SLA2 and with the phosphorylated C-terminus of SH2B2. Interacts with EGFR, SYK and ZAP70 via the highly conserved Cbl-N region. Also interacts with SORBS1 and INPPL1/SHIP2. Interacts with phosphorylated LAT2. May interact with CBLB.,
Research Area
ErbB_HER;Ubiquitin mediated proteolysis;Endocytosis;Jak_STAT;T_Cell_Receptor;Insulin_Receptor;Pathways in cancer;Chronic myeloid leukemia;