Summary
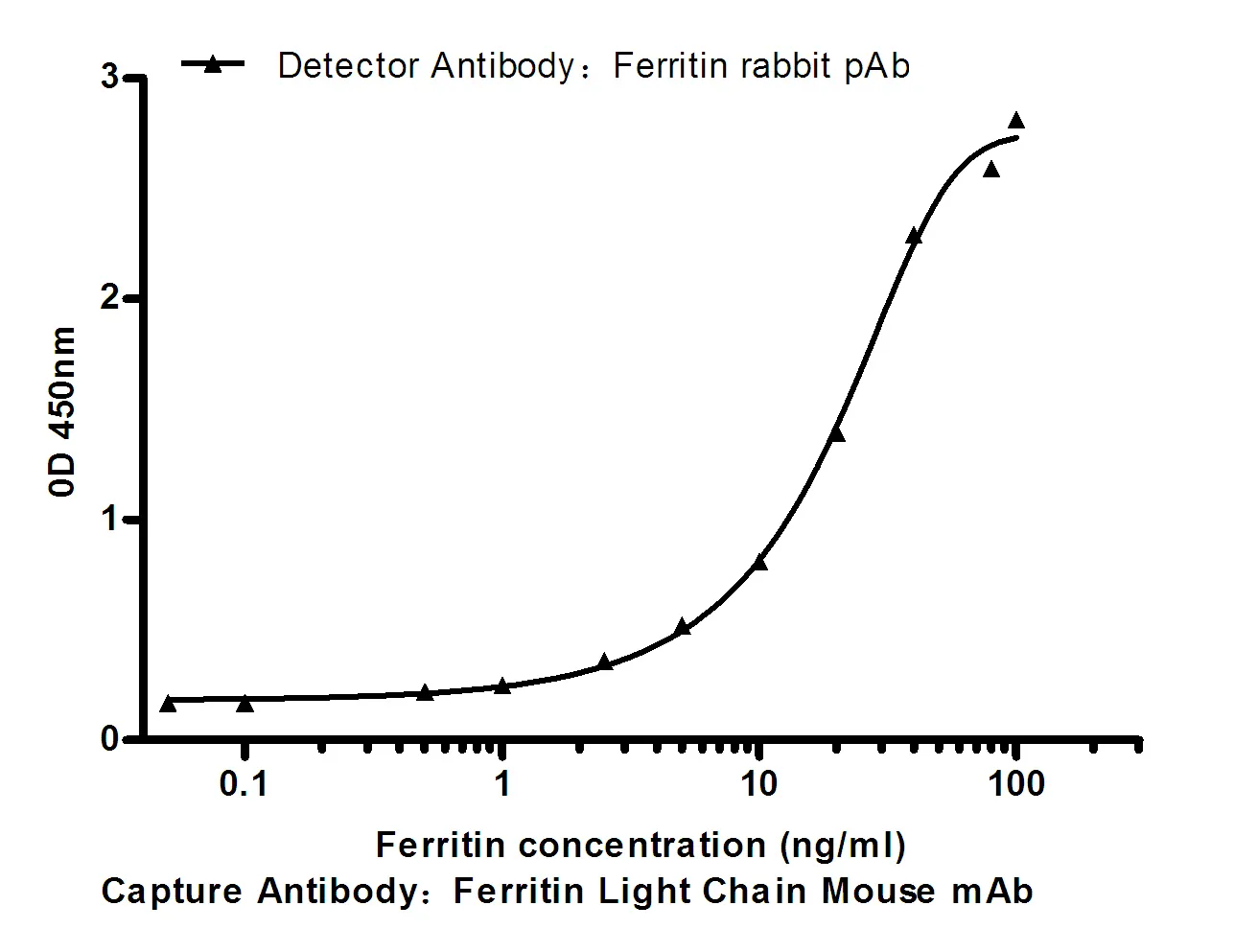
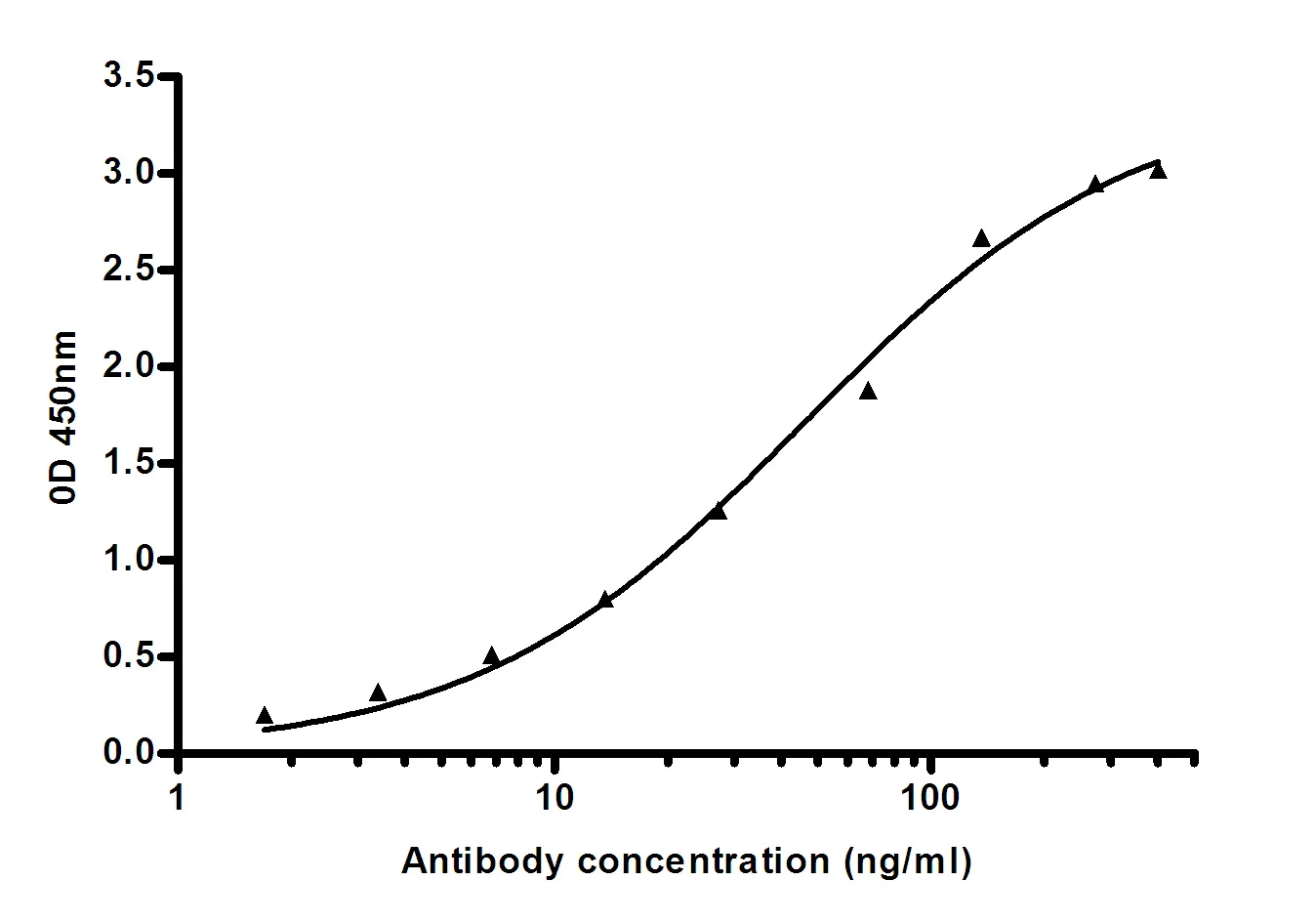
Performance
Immunogen
Application
Background
The protein encoded by this gene is a membrane glycoprotein that mediates cell-cell signaling and plays a critical role in the growth and development of multiple organ systems. An extraordinary variety of different isoforms are produced from this gene through alternative promoter usage and splicing. These isoforms are expressed in a tissue-specific manner and differ significantly in their structure, and are classified as types I, II, III, IV, V and VI. Dysregulation of this gene has been linked to diseases such as cancer, schizophrenia, and bipolar disorder (BPD). [provided by RefSeq, Apr 2016],alternative products:Additional isoforms seem to exist. Isoforms have been classified as type I NRGs (isoforms with an Ig domain and a glycosylation domain, isoforms 1-8), type II NRGs (isoforms with an Ig domain but no glycosylation domain, isoform 9) and type III NRGs (isoforms with a Cys-rich domain, isoform 10). All these isoforms perform distinct tissue-specific functions,developmental stage:Detectable at early embryonic ages.,disease:A rare chromosomal aberration involving NRG1 produces gamma-heregulin. Translocation t(8;11) with ODZ4. The translocation fuses the 5'-end of ODZ4 to NRG1 (isoform 8). The product of this translocation was first thought to be an alternatively spliced isoform. Gamma-heregulin is a soluble activating ligand for the ERBB2-ERBB3 receptor complex and acts as an autocrine growth factor in a specific breast cancer cell line (MDA-MB-175). Not detected in breast carcinoma samples, including ductal, lobular, medullary, and mucinous histological types, neither in other breast cancer cell lines.,domain:ERBB receptor binding is elicited entirely by the EGF-like domain.,domain:The cytoplasmic domain may be involved in the regulation of trafficking and proteolytic processing. Regulation of the proteolytic processing involves initial intracellular domain dimerization.,function:Direct ligand for ERBB3 and ERBB4 tyrosine kinase receptors. Concomitantly recruits ERBB1 and ERBB2 coreceptors, resulting in ligand-stimulated tyrosine phosphorylation and activation of the ERBB receptors. The multiple isoforms perform diverse functions such as inducing growth and differentiation of epithelial, glial, neuronal, and skeletal muscle cells; inducing expression of acetylcholine receptor in synaptic vessicles during the formation of the neuromuscular junction; stimulating lobuloalveolar budding and milk production in the mammary gland and inducing differentiation of mammary tumor cells; stimulating Schwann cell proliferation; implication in the development of the myocardium such as trabeculation of the developing heart.,PTM:Extensive glycosylation precedes the proteolytic cleavage.,PTM:Proteolytic cleavage close to the plasma membrane on the external face leads to the release of the soluble growth factor form.,similarity:Belongs to the neuregulin family.,similarity:Contains 1 EGF-like domain.,similarity:Contains 1 Ig-like C2-type (immunoglobulin-like) domain.,subcellular location:Does not seem to be active.,subcellular location:Has a signal peptide.,subcellular location:May be nuclear.,subunit:The cytoplasmic domain interacts with the LIM domain region of LIMK1.,tissue specificity:Type I isoforms are the predominant forms expressed in the endocardium. Isoform alpha is expressed in breast, ovary, testis, prostate, heart, skeletal muscle, lung, placenta liver, kidney, salivary gland, small intestine and brain, but not in uterus, stomach, pancreas, and spleen. Isoform 3 is the predominant form in mesenchymal cells and in non-neuronal organs, whereas isoform 5 is the major neuronal form. Isoform 8 is expressed in spinal cord and brain. Isoform 9 is the major form in skeletal muscle cells; in the nervous system it is expressed in spinal cord and brain. Also detected in adult heart, placenta, lung, liver, kidney, and pancreas.,
Research Area