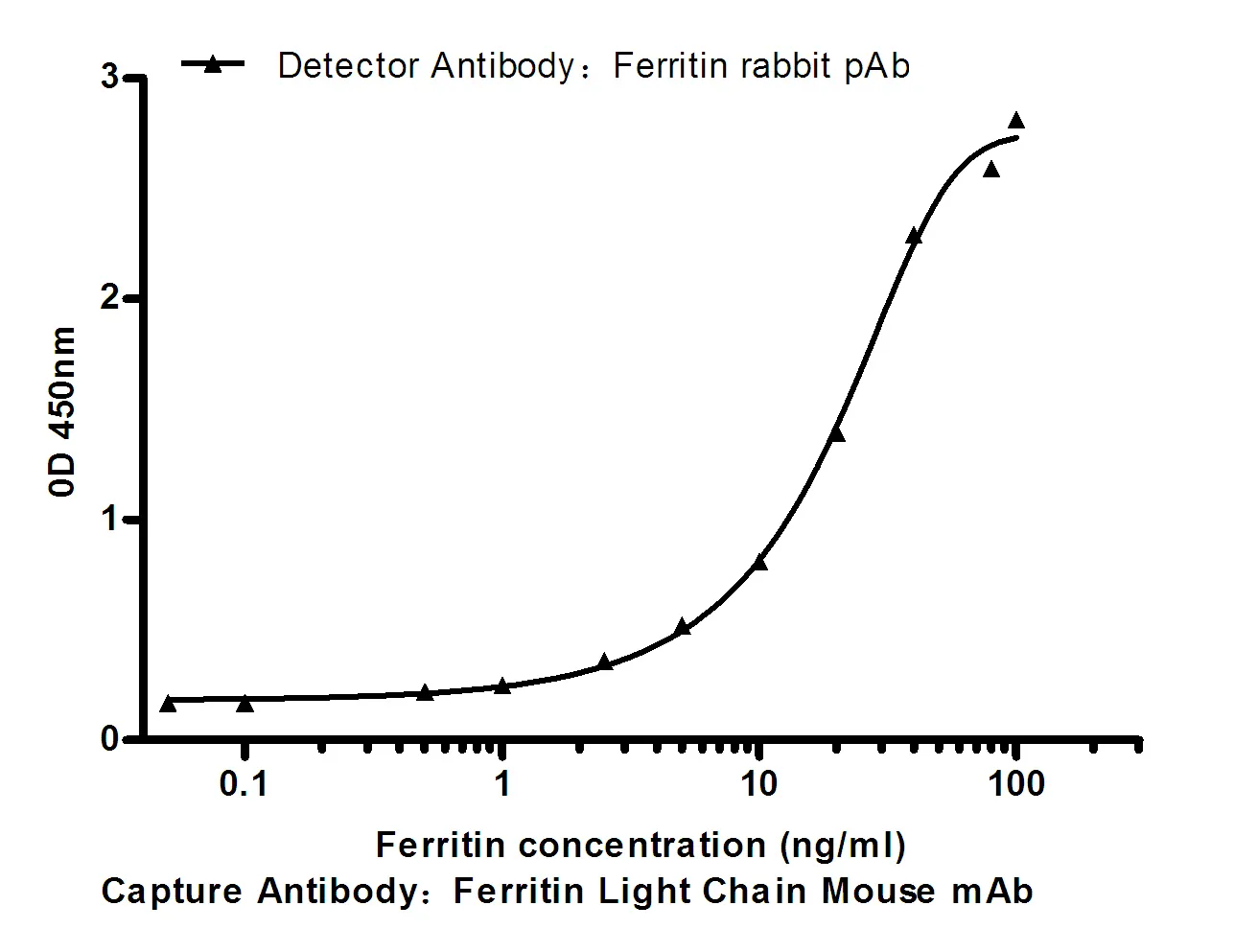
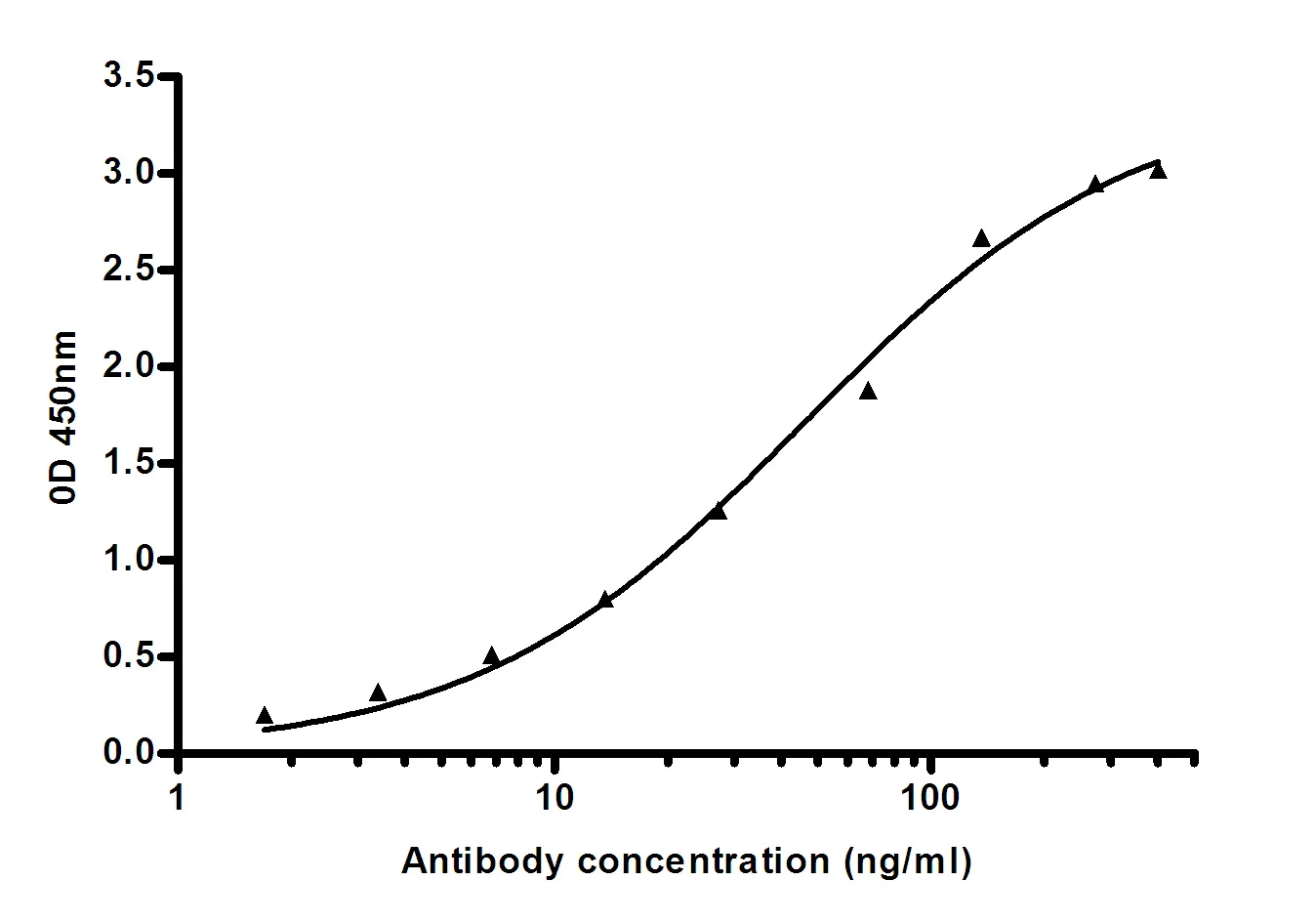
Summary
Performance
Immunogen
Application
Background
myosin VI(MYO6) Homo sapiens This gene encodes a reverse-direction motor protein that moves toward the minus end of actin filaments and plays a role in intracellular vesicle and organelle transport. The protein consists of a motor domain containing an ATP- and an actin-binding site and a globular tail which interacts with other proteins. This protein maintains the structural integrity of inner ear hair cells and mutations in this gene cause non-syndromic autosomal dominant and recessive hearing loss. Alternative splicing results in multiple transcript variants encoding distinct isoforms. [provided by RefSeq, Jul 2014],disease:Defects in MYO6 are the cause of non-syndromic sensorineural deafness autosomal dominant type 22 (DFNA22) [MIM:606346]. DFNA22 is a form of sensorineural hearing loss. Sensorineural deafness results from damage to the neural receptors of the inner ear, the nerve pathways to the brain, or the area of the brain that receives sound information. DFNA22 is progressive and postlingual, with onset during childhood. By the age of approximately 50 years, affected individuals invariably have profound sensorineural deafness.,disease:Defects in MYO6 are the cause of non-syndromic sensorineural deafness autosomal recessive type 37 (DFNB37) [MIM:607821].,disease:Defects in MYO6 are the cause of sensorineural deafness with hypertrophic cardiomyopathy (DFNHCM) [MIM:606346].,domain:Divided into three regions: a N-terminal motor (head) domain, followed by a neck domain consisting of a calmodulin-binding linker domain and a single IQ motif, and a C-terminal tail region with a coiled-coil and a unique globular domain required for interaction with other proteins.,function:Myosins are actin-based motor molecules with ATPase activity. Unconventional myosins serve in intracellular movements. Myosin 6 is a reverse-direction motor protein that moves towards the minus-end of actin filaments. Has slow rate of actin-activated ADP release due to weak ATP binding. Functions in a variety of intracellular processes such as vesicular membrane trafficking and cell migration. Required for the structural integrity of the Golgi apparatus via the p53-dependent pro-survival pathway. Appears to be involved in a very early step of clathrin-mediated endocytosis in polarized epithelial cells. May act as a regulator of F-actin dynamics. May play a role in transporting DAB2 from the plasma membrane to specific cellular targets. Required for structural integrity of inner ear hair cells.,PTM:Phosphorylation in the motor domain, induced by EGF, results in translocation of MYO6 from the cell surface to membrane ruffles and affects F-actin dynamics. Phosphorylated in vitro by p21-activated kinase (PAK).,similarity:Contains 1 IQ domain.,similarity:Contains 1 myosin head-like domain.,subcellular location:Also present in endocyctic vesicles, and membrane ruffles. Translocates from membrane ruffles, endocytic vesicles and cytoplasm to Golgi apparatus, perinuclear membrane and nucleus through induction by p53 and p53-induced DNA damage. Recruited into membrane ruffles from cell surface by EGF-stimulation. Colocalizes with DAB2 in clathrin-coated pits/vesicles.,subunit:Homodimer. Binding to calmodulin through a unique insert, not found in other myosins, located in the neck region between the motor domain and the IQ domain appears to contribute to the directionality reversal. This interaction occurs only if the C-terminal lobe of calmodulin is occupied by calcium. Interaction with F-actin/ACTN1 occurs only at the apical brush border domain of the proximal tubule cells (By similarity). Interacts with DAB2. In vitro, the C-terminal globular tail binds a C-terminal region of DAB2. Interacts with CFTR. Forms a complex with CFTR and DAB2 in the apical membrane of epithelial cells.,tissue specificity:Expressed in most tissues examined including heart, brain, placenta, pancreas, spleen, thymus, prostate, testis, ovary, small intestine and colon. Highest levels in brain, pancreas, testis and small intestine. Also expressed in fetal brain and cochlea. Isoform 1 and isoform 2, containing the small insert, and isoform 4, containing neither insert, are expressed in unpolarized epithelial cells.,
Research Area