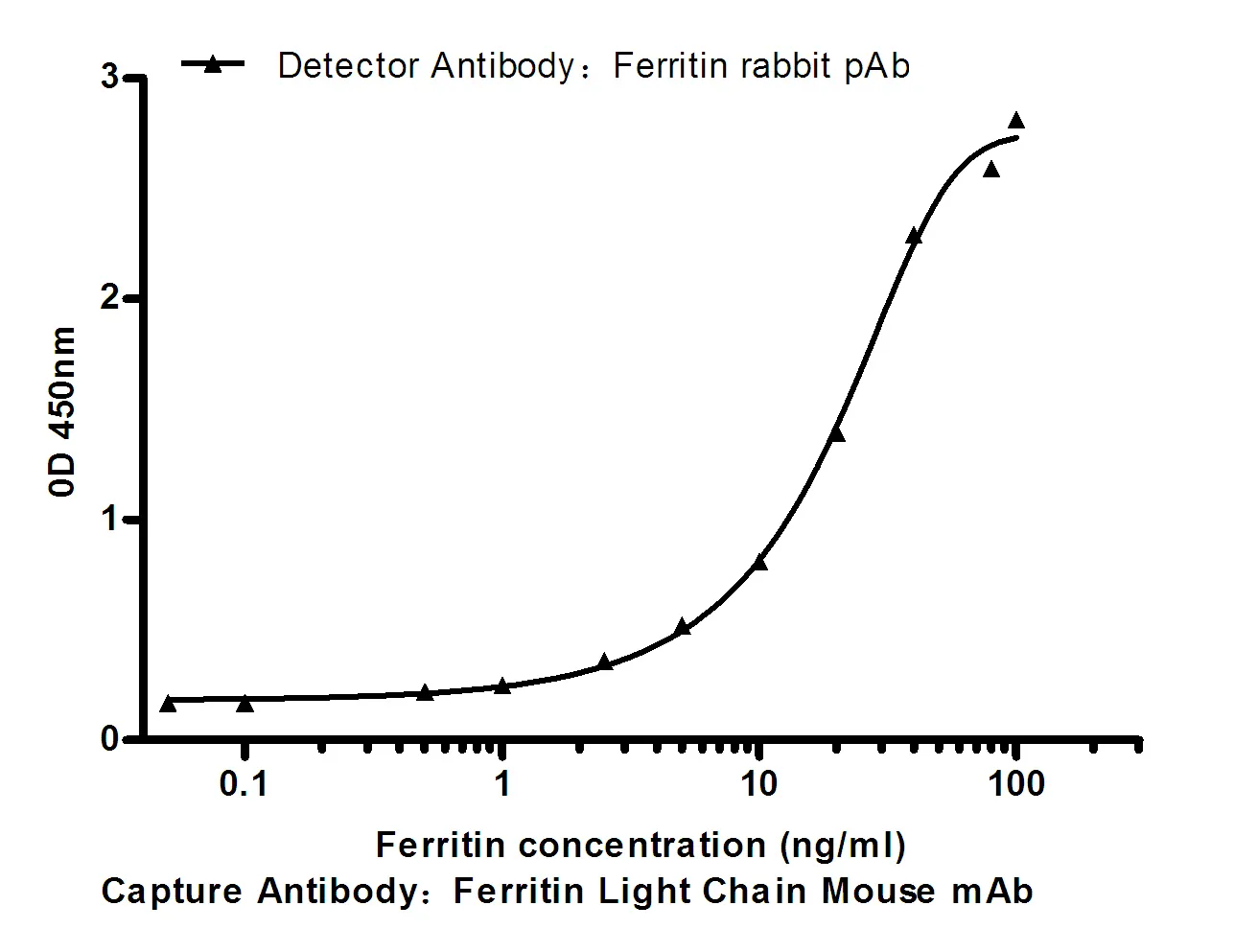
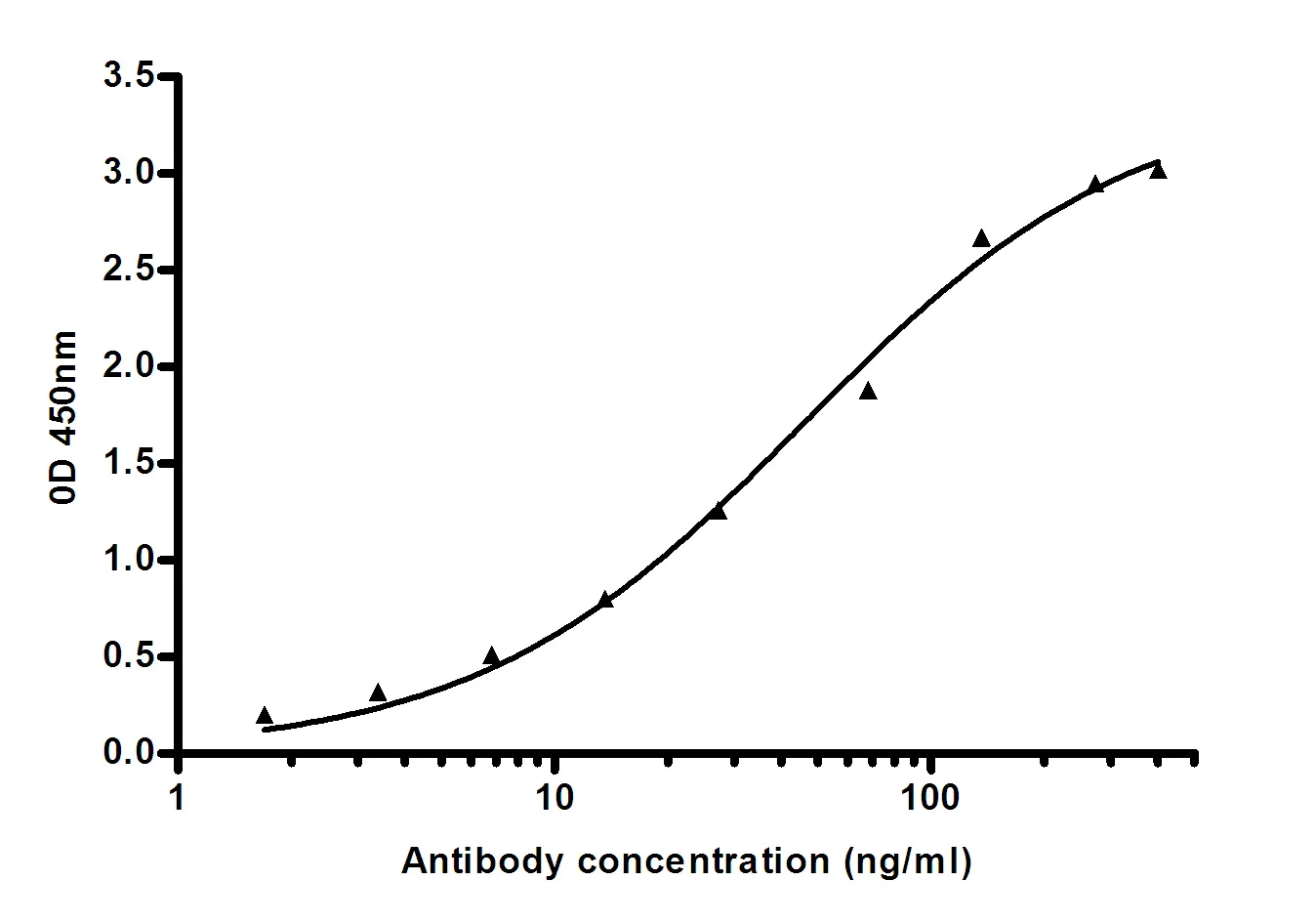
Summary
Performance
Immunogen
Application
Background
BCL2, apoptosis regulator(BCL2) Homo sapiens This gene encodes an integral outer mitochondrial membrane protein that blocks the apoptotic death of some cells such as lymphocytes. Constitutive expression of BCL2, such as in the case of translocation of BCL2 to Ig heavy chain locus, is thought to be the cause of follicular lymphoma. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Feb 2016],disease:A chromosomal aberration involving BCL2 may be a cause of follicular lymphoma (FL) [MIM:151430]; also known as type II chronic lymphatic leukemia. Translocation t(14;18)(q32;q21) with immunoglobulin gene regions. BCL2 mutations found in non-Hodgkin lymphomas carrying the chromosomal translocation could be attributed to the Ig somatic hypermutation mechanism resulting in nucleotide transitions.,domain:The BH4 motif is required for anti-apoptotic activity and for interaction with RAF-1.,function:Suppresses apoptosis in a variety of cell systems including factor-dependent lymphohematopoietic and neural cells. Regulates cell death by controlling the mitochondrial membrane permeability. Appears to function in a feedback loop system with caspases. Inhibits caspase activity either by preventing the release of cytochrome c from the mitochondria and/or by binding to the apoptosis-activating factor (APAF-1).,online information:Bcl-2 entry,PTM:Phosphorylation/dephosphorylation on Ser-70 regulates anti-apoptotic activity. Growth factor-stimulated phosphorylation on Ser-70 by PKC is required for the anti-apoptosis activity and occurs during the G2/M phase of the cell cycle. In the absence of growth factors, BCL2 appears to be phosphorylated by other protein kinases such as ERKs and stress-activated kinases. Dephosphorylated by protein phosphatase 2A (PP2A).,PTM:Proteolytically cleaved by caspases during apoptosis. The cleaved protein, lacking the BH4 motif, has pro-apoptotic activity, causes the release of cytochrome c into the cytosol promoting further caspase activity.,similarity:Belongs to the Bcl-2 family.,subunit:Forms homodimers, and heterodimers with BAX, BAD, BAK and Bcl-X(L). Heterodimerization with BAX requires intact BH1 and BH2 motifs, and is necessary for anti-apoptotic activity (By similarity). Also interacts with APAF1, RAF-1, TP53BP2, BBC3, BCL2L1, MRPL41 and BNIPL. Binding to FKBP8 seems to target BCL2 to the mitochondria and probably interferes with the binding of BCL2 to its targets.,tissue specificity:Expressed in a variety of tissues.,
Research Area
Apoptosis_Inhibition;Apoptosis_Mitochondrial;Apoptosis_Overview;Focal adhesion;Neurotrophin;Amyotrophic lateral sclerosis (ALS);Pathways in cancer;Colorectal cancer;Prostate cancer;Small cell lung cancer;