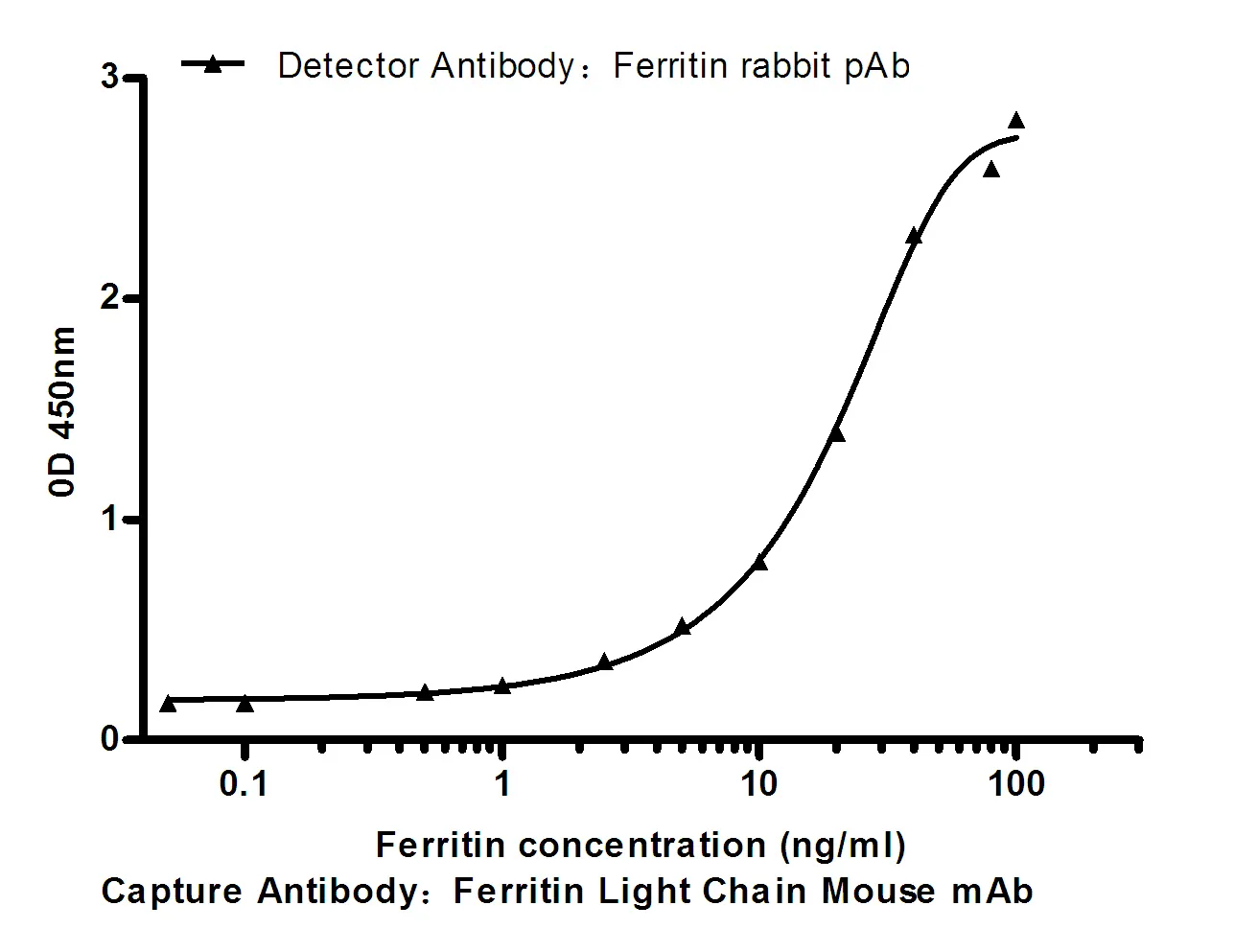
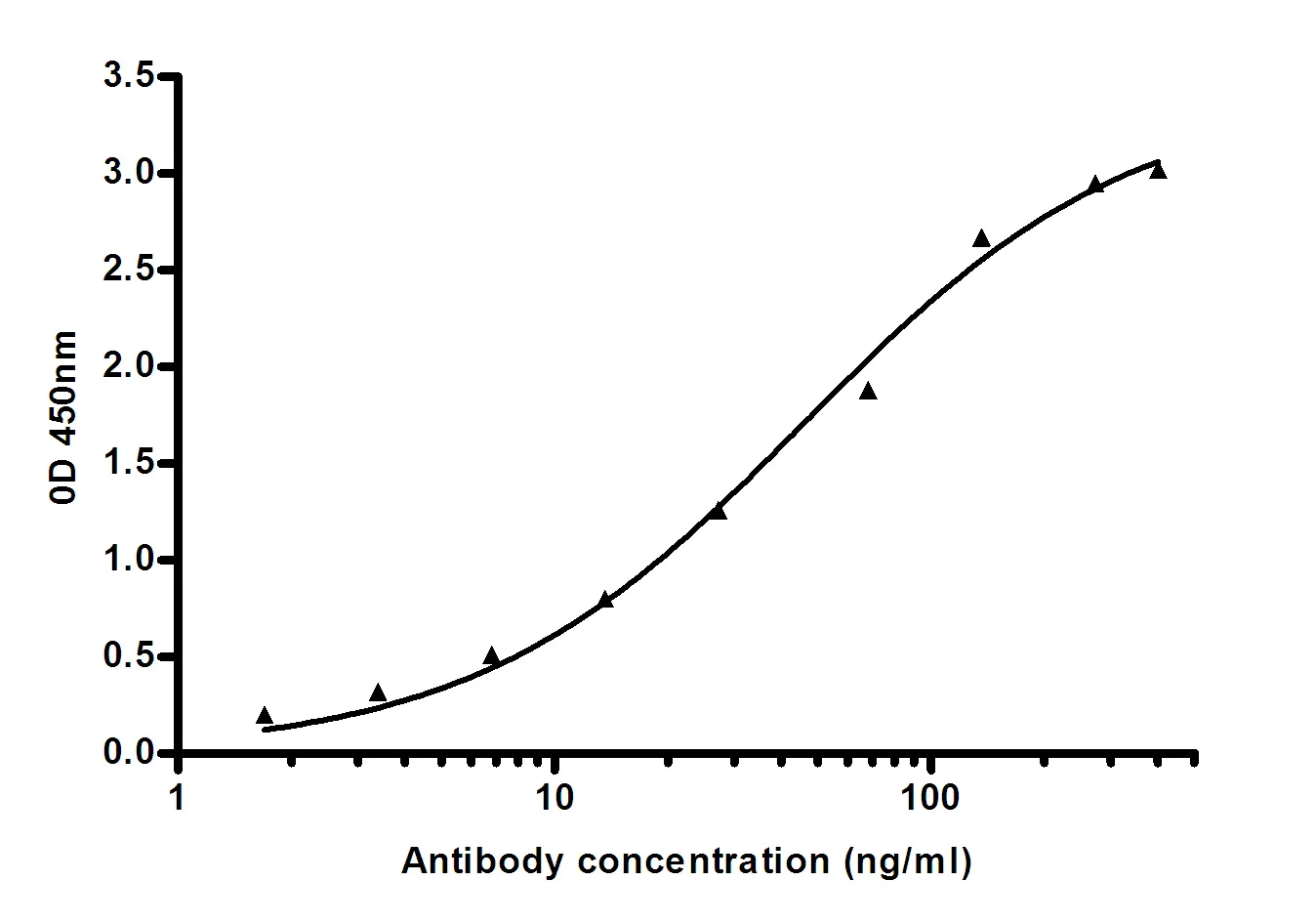
Summary
Performance
Immunogen
Application
Background
This gene encodes a highly conserved preproprotein that is proteolytically processed to generate four main cleavage products including saposins A, B, C, and D. Each domain of the precursor protein is approximately 80 amino acid residues long with nearly identical placement of cysteine residues and glycosylation sites. Saposins A-D localize primarily to the lysosomal compartment where they facilitate the catabolism of glycosphingolipids with short oligosaccharide groups. The precursor protein exists both as a secretory protein and as an integral membrane protein and has neurotrophic activities. Mutations in this gene have been associated with Gaucher disease and metachromatic leukodystrophy. Alternative splicing results in multiple transcript variants, at least one of which encodes an isoform that is proteolytically processed. [provided by RefSeq, Feb 2016],alternative products:Additional isoforms seem to exist,disease:Defects in PSAP are the cause of combined saposin deficiency (CSAPD) [MIM:611721]; also known as prosaposin deficiency. CSAPD is due to absence of all saposins, leading to a fatal storage disorder with hepatosplenomegaly and severe neurological involvement.,disease:Defects in PSAP saposin-A region are the cause of atypical Krabbe disease (AKRD) [MIM:611722]. AKRD is a disorder of galactosylceramide metabolism. AKRD features include progressive encephalopathy and abnormal myelination in the cerebral white matter resembling Krabbe disease.,disease:Defects in PSAP saposin-B region are the cause of a variant of metachromatic leukodystrophy (MLD) [MIM:249900].,disease:Defects in PSAP saposin-C region are the cause of atypical Gaucher disease (AGD) [MIM:610539]. Affected individuals have marked glucosylceramide accumulation in the spleen without having a deficiency of glucosylceramide-beta glucosidase characteristic of classic Gaucher disease, a lysosomal storage disorder.,disease:Defects in PSAP saposin-D region are the cause of a variant of Tay-Sachs disease (GM2-gangliosidosis).,function:Saposin-A and saposin-C stimulate the hydrolysis of glucosylceramide by beta-glucosylceramidase (EC 3.2.1.45) and galactosylceramide by beta-galactosylceramidase (EC 3.2.1.46). Saposin-C apparently acts by combining with the enzyme and acidic lipid to form an activated complex, rather than by solubilizing the substrate.,function:Saposin-B stimulates the hydrolysis of galacto-cerebroside sulfate by arylsulfatase A (EC 3.1.6.8), GM1 gangliosides by beta-galactosidase (EC 3.2.1.23) and globotriaosylceramide by alpha-galactosidase A (EC 3.2.1.22). Saposin-B forms a solubilizing complex with the substrates of the sphingolipid hydrolases.,function:Saposin-D is a specific sphingomyelin phosphodiesterase activator (EC 3.1.4.12).,function:The lysosomal degradation of sphingolipids takes place by the sequential action of specific hydrolases. Some of these enzymes require specific low-molecular mass, non-enzymic proteins: the sphingolipids activator proteins (coproteins).,miscellaneous:Saposin-B co-purifies with 1 molecule of phosphatidylethanolamine.,PTM:N-linked glycans show a high degree of microheterogeneity.,PTM:The one residue extended Saposin-B-Val is only found in 5% of the chains.,PTM:This precursor is proteolytically processed to 4 small peptides, which are similar to each other and are sphingolipid hydrolase activator proteins.,similarity:Contains 2 saposin A-type domains.,similarity:Contains 4 saposin B-type domains.,subunit:Saposin-B is a homodimer.,
Research Area
Lysosome;