Summary
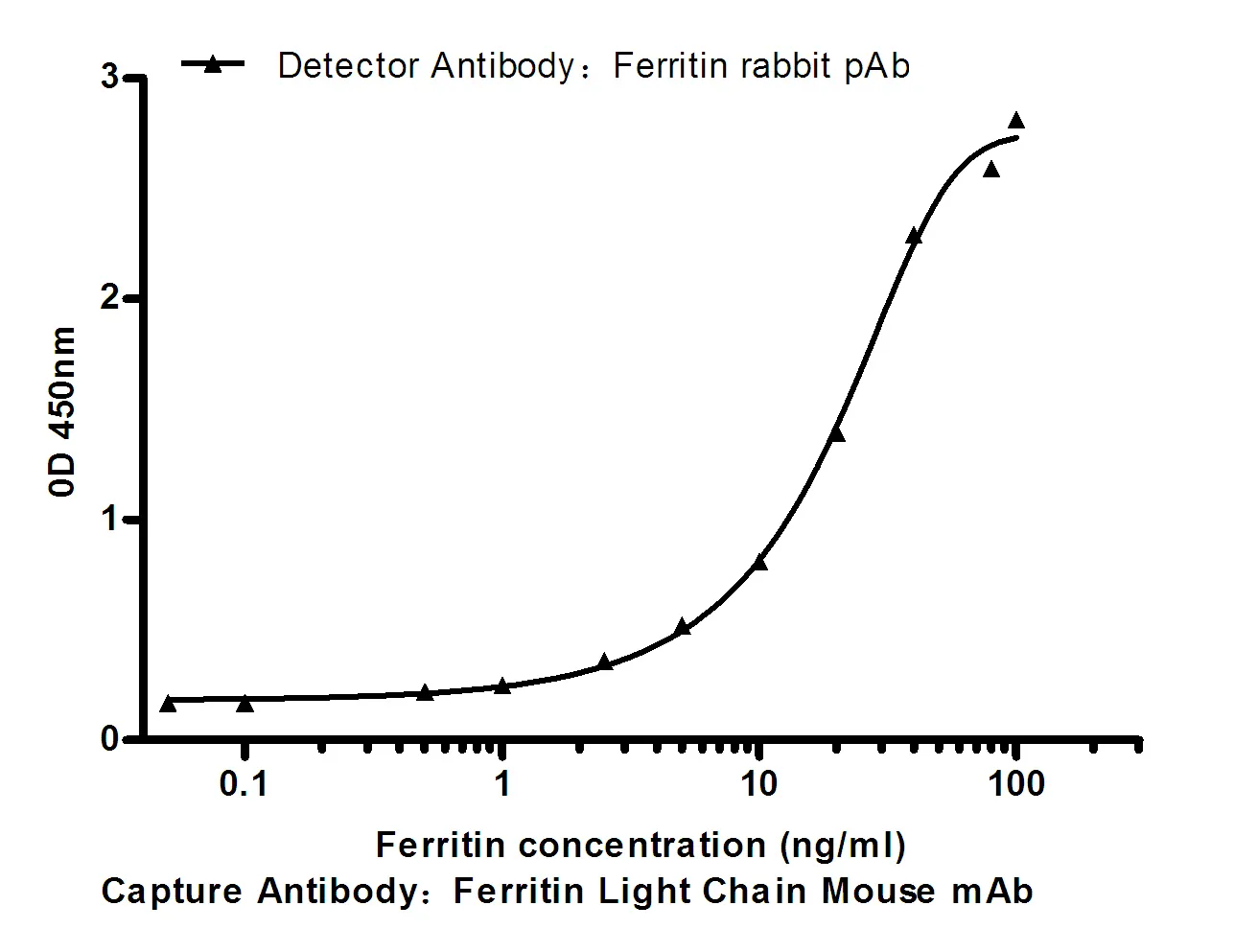
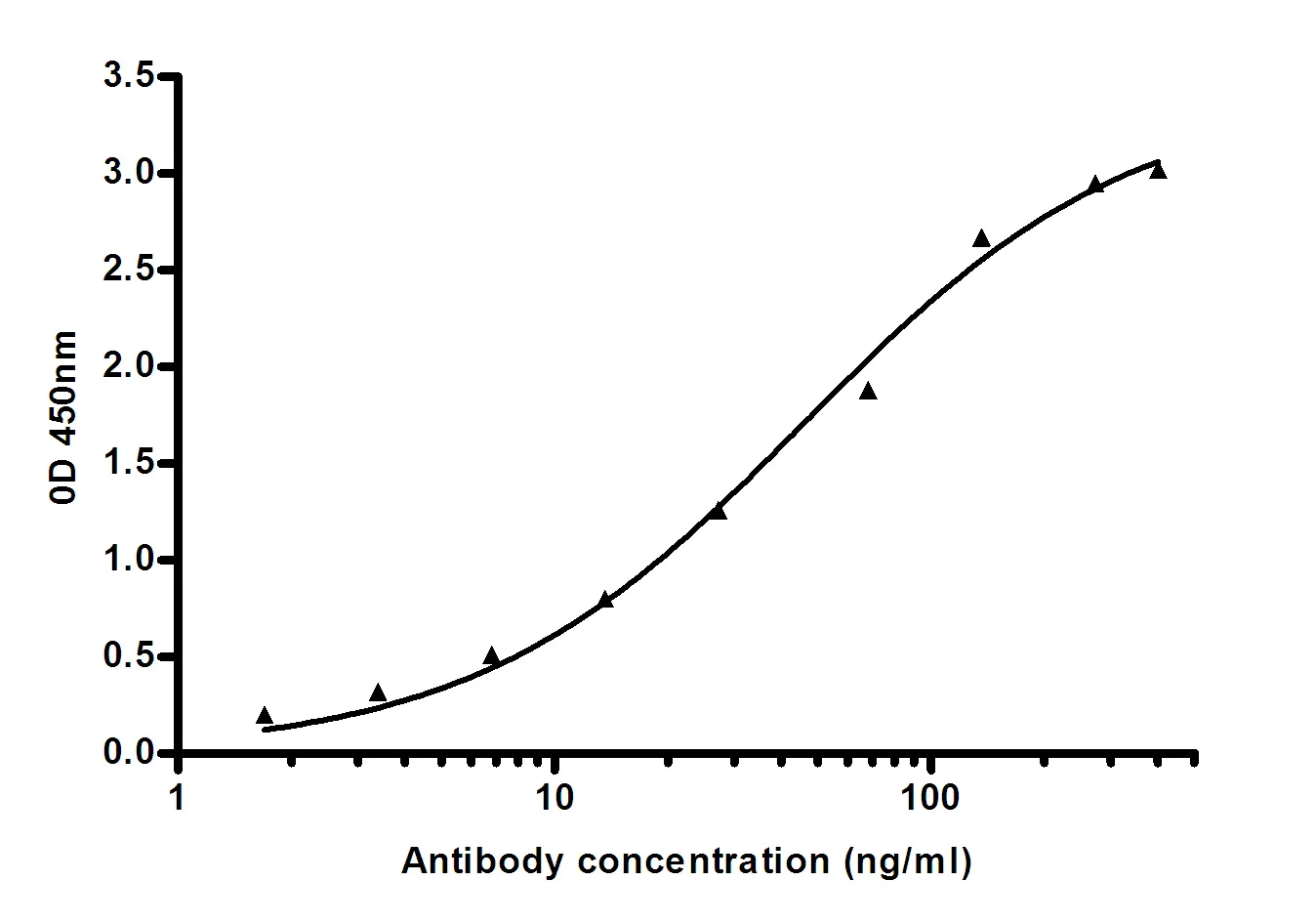
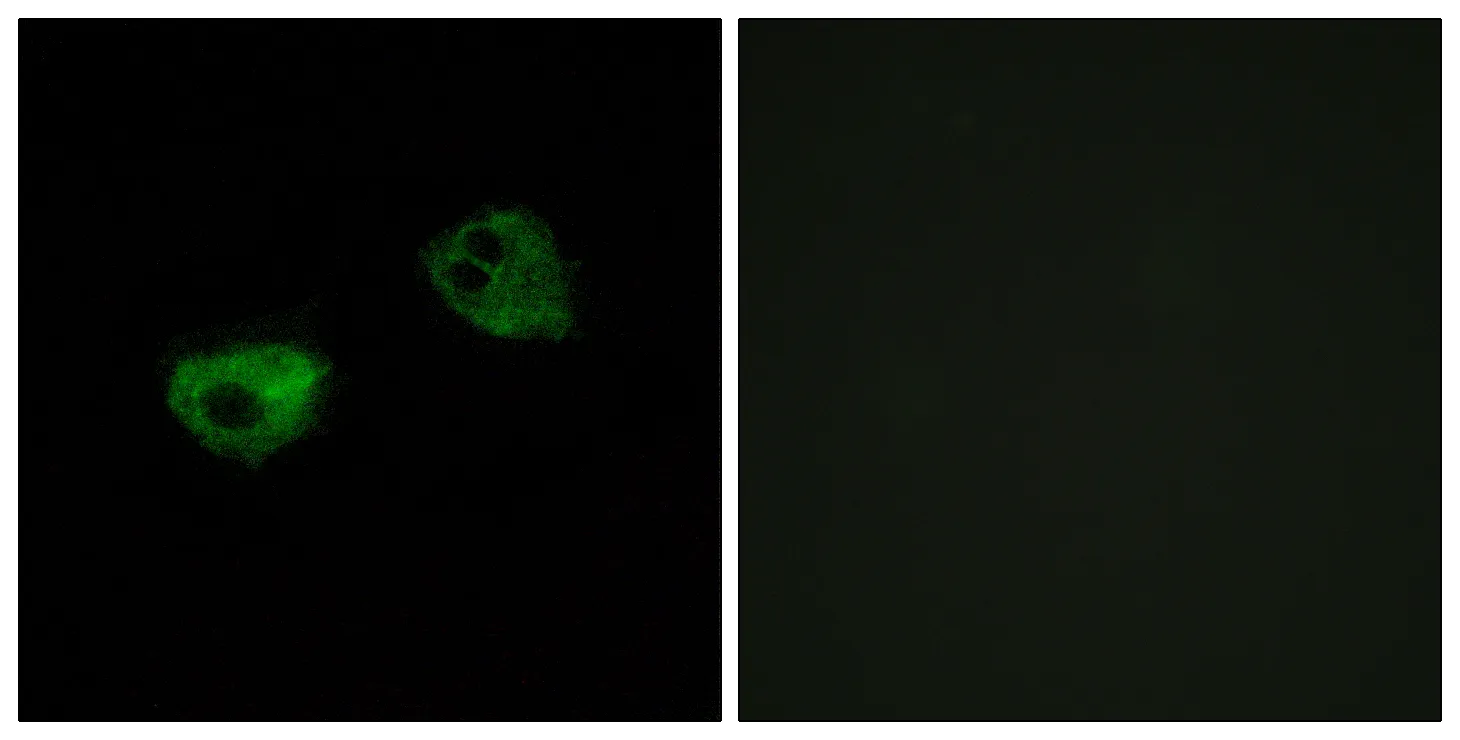
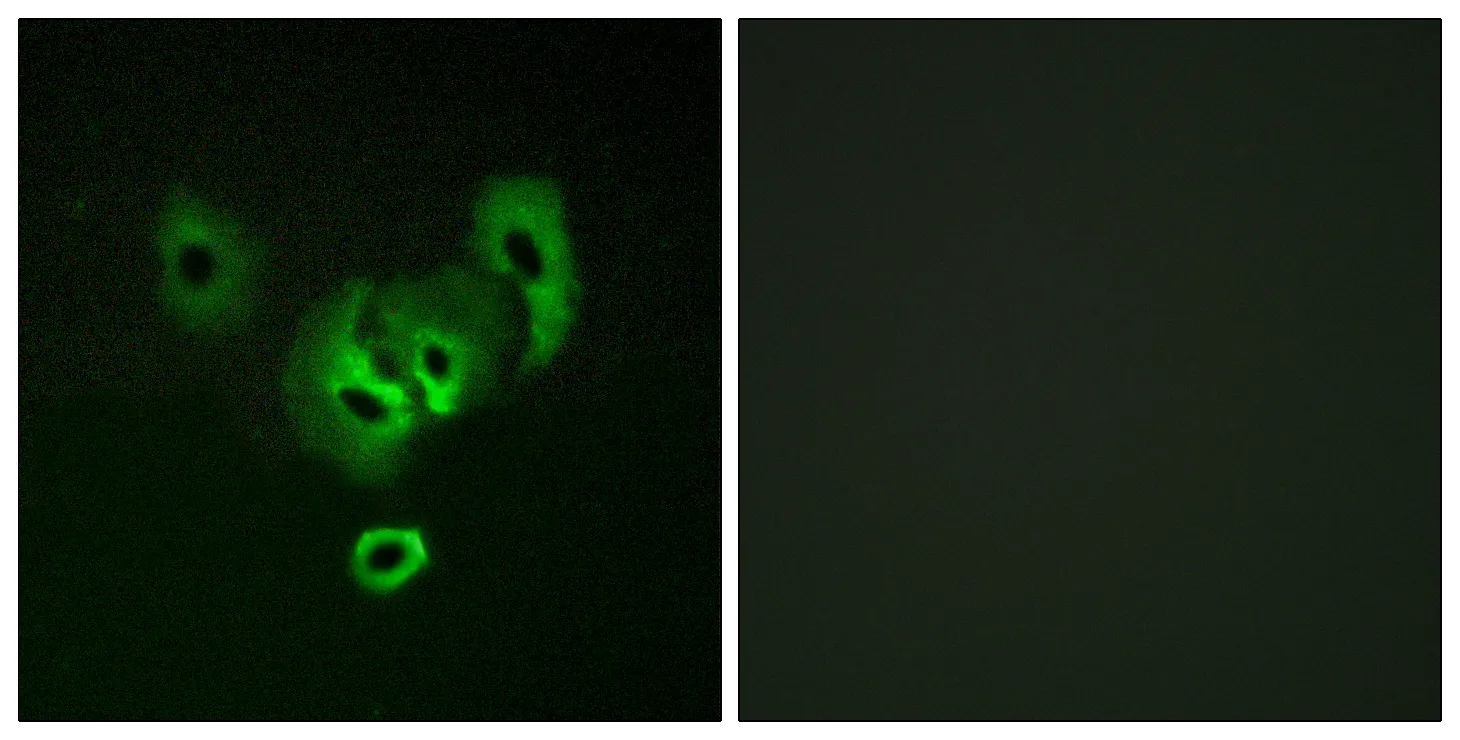
Performance
Immunogen
Application
Background
This gene encodes one of the six subunits of type IV collagen, the major structural component of basement membranes. Mutations in this gene are associated with X-linked Alport syndrome, also known as hereditary nephritis. Like the other members of the type IV collagen gene family, this gene is organized in a head-to-head conformation with another type IV collagen gene so that each gene pair shares a common promoter. Alternatively spliced transcript variants have been identified for this gene. [provided by RefSeq, Aug 2010],disease:Defects in COL4A5 are the cause of Alport syndrome X-linked (APSX) [MIM:301050]. APSX is characterized by progressive glomerulonephritis, renal failure, sensorineural deafness, specific eye abnormalities (lenticonous and macular flecks), and glomerular basement membrane defects. The disorder shows considerable heterogeneity in that families differ in the age of end-stage renal disease and the occurrence of deafness.,disease:Deletions covering the N-terminal regions of COL4A5 and COL4A6, which are localized in a head-to-head manner, are the cause of diffuse leiomyomatosis with Alport syndrome (DL-ATS) [MIM:308940]; also known as esophageal and vulval leiomyomatosis with nephropathy or Alport syndrome and diffuse leiomyomatosis (ATS-DL). DL-ATS is the combination of AS and diffuse leiomyomatosis (DL). DL is a tumorous process involving smooth muscle cells, mostly of the esophagus, but also of the tracheobronchial tree and the female genital tract.,domain:Alpha chains of type IV collagen have a non-collagenous domain (NC1) at their C-terminus, frequent interruptions of the G-X-Y repeats in the long central triple-helical domain (which may cause flexibility in the triple helix), and a short N-terminal triple-helical 7S domain.,function:Type IV collagen is the major structural component of glomerular basement membranes (GBM), forming a 'chicken-wire' meshwork together with laminins, proteoglycans and entactin/nidogen.,PTM:Prolines at the third position of the tripeptide repeating unit (G-X-Y) are hydroxylated in some or all of the chains.,PTM:Type IV collagens contain numerous cysteine residues which are involved in inter- and intramolecular disulfide bonding. 12 of these, located in the NC1 domain, are conserved in all known type IV collagens.,similarity:Belongs to the type IV collagen family.,similarity:Contains 1 collagen IV NC1 (C-terminal non-collagenous) domain.,subunit:There are six type IV collagen isoforms, alpha 1(IV)-alpha 6(IV), each of which can form a triple helix structure with 2 other chains to generate type IV collagen network.,
Research Area