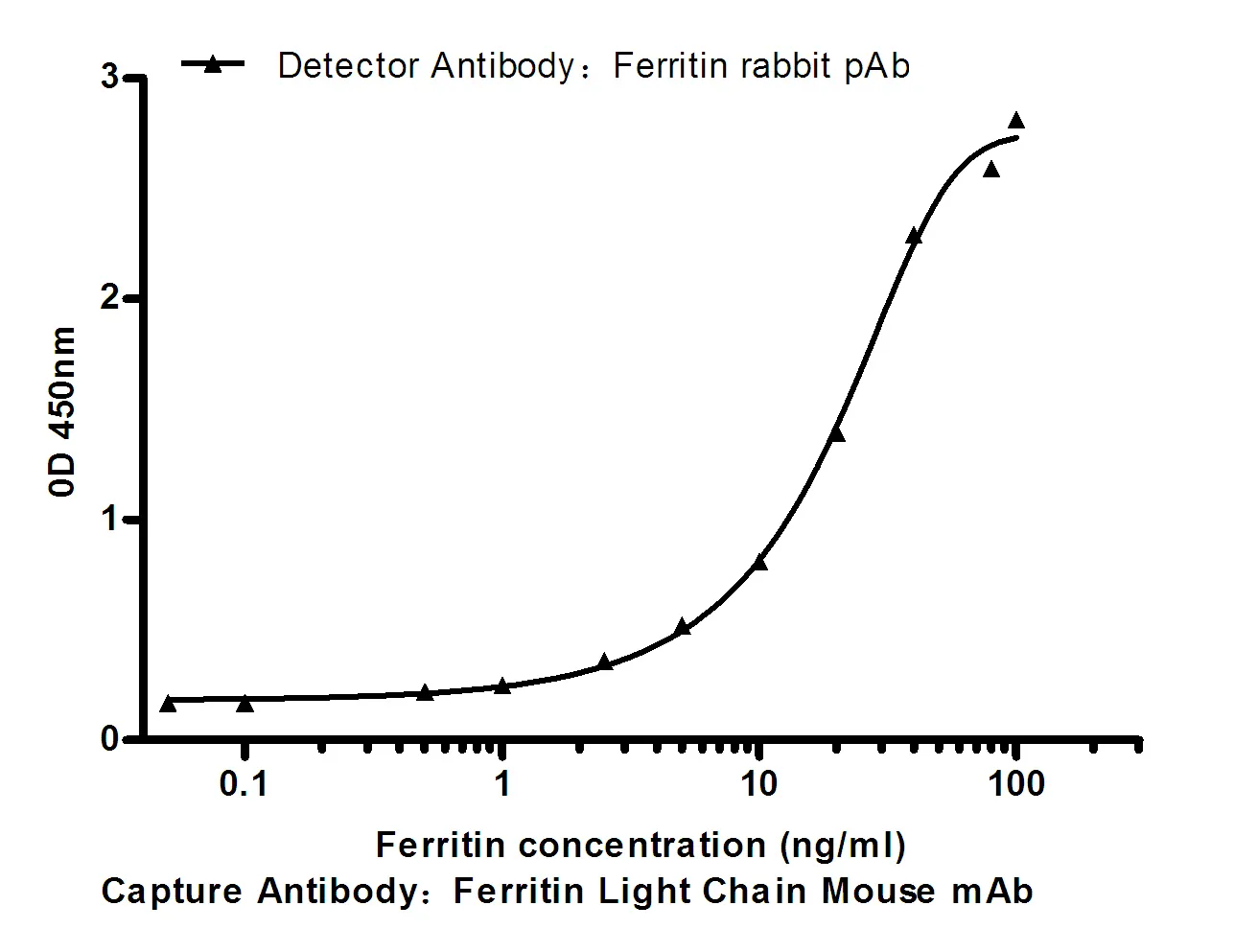
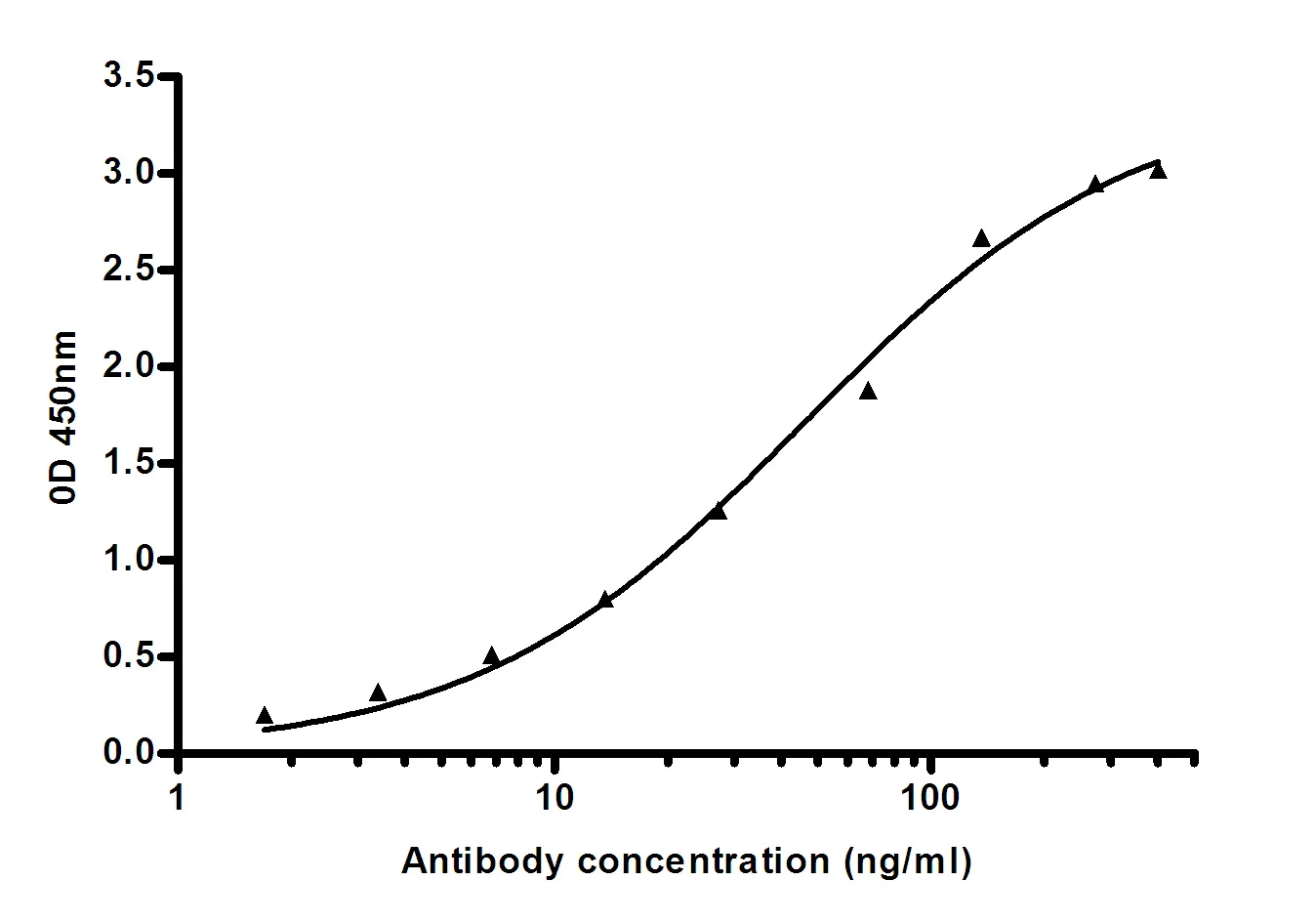
Summary
Performance
Immunogen
Application
Background
domain:The fifth SH3 domain mediates binding with ADAM12, ADAM15 and ADAM19.,domain:The PX domain is required for podosome localization, and for binding phosphatidylinositol 3-phosphate (PtdIns(3)P) and phosphatidylinositol 3,4-biphosphate (PtdIns(3,4)P2).,function:Required for podosome formation, degradation of the extracellular matrix, and for the invasiveness of some cancer cells. Binds phosphatidylinositol 3-phosphate (PtdIns(3)P) and phosphatidylinositol 3,4-biphosphate (PtdIns(3,4)P2). In association with ADAM12, mediates the neurotoxic effect of beta-amyloid peptide.,PTM:Tyrosine phosphorylated by SRC. Phosphorylation plays a regulatory role in the protein localization. The intramolecular interaction of the PX domain with the third SH3 domain maintains the protein in the cytoplasm and phosphorylation disrupts this interaction, resulting in the redistribution of the protein from cytoplasm to the perimembrane region. Phosphorylated on serine upon DNA damage, probably by ATM or ATR.,similarity:Belongs to the SH3PXD2 family.,similarity:Contains 1 PX (phox homology) domain.,similarity:Contains 5 SH3 domains.,subcellular location:Cytoplasmic in normal cells and localizes to podosomes in SRC-transformed cells.,subunit:Interacts with ADAM12, ADAM15 and ADAM19.,tissue specificity:Found in several cancer cell lines, particularly invasive breast carcinomas and melanomas.,domain:The fifth SH3 domain mediates binding with ADAM12, ADAM15 and ADAM19.,domain:The PX domain is required for podosome localization, and for binding phosphatidylinositol 3-phosphate (PtdIns(3)P) and phosphatidylinositol 3,4-biphosphate (PtdIns(3,4)P2).,function:Required for podosome formation, degradation of the extracellular matrix, and for the invasiveness of some cancer cells. Binds phosphatidylinositol 3-phosphate (PtdIns(3)P) and phosphatidylinositol 3,4-biphosphate (PtdIns(3,4)P2). In association with ADAM12, mediates the neurotoxic effect of beta-amyloid peptide.,PTM:Tyrosine phosphorylated by SRC. Phosphorylation plays a regulatory role in the protein localization. The intramolecular interaction of the PX domain with the third SH3 domain maintains the protein in the cytoplasm and phosphorylation disrupts this interaction, resulting in the redistribution of the protein from cytoplasm to the perimembrane region. Phosphorylated on serine upon DNA damage, probably by ATM or ATR.,similarity:Belongs to the SH3PXD2 family.,similarity:Contains 1 PX (phox homology) domain.,similarity:Contains 5 SH3 domains.,subcellular location:Cytoplasmic in normal cells and localizes to podosomes in SRC-transformed cells.,subunit:Interacts with ADAM12, ADAM15 and ADAM19.,tissue specificity:Found in several cancer cell lines, particularly invasive breast carcinomas and melanomas.,
Research Area